IF
in JCR CiteScore Rank About IJO Current Issue Featured
Articles Articles
In Press Recent
Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1361-1369
·Basic Research·
The ocular toxicity and pharmacokinetics of
simvastatin following intravitreal injection in mice
Dennis Y. Tse1,2, Seong Jae Kim3,
Inyoung Chung3, Feng He4, Theodore G. Wensel4,
Samuel M. Wu1
1Department of Ophthalmology, Baylor
College of Medicine, Houston, TX 77030, USA
2School of Optometry, the Hong Kong
Polytechnic University, Hong Kong, China
3Department of Ophthalmology, Institute of
Health Sciences, College of Medicine, Gyeongsang National University, Jinju
52727, Korea
4Department of Biochemistry and Molecular
Biology, Baylor College of Medicine, Houston, TX 77030, USA
Co-first authors: Dennis Y. Tse, Seong
Jae Kim and Inyoung Chung
Correspondence to: Inyoung Chung.
Department of Ophthalmology, College of Medicine, Gyeongsang National
University, Jinju 52727, Korea. inyoung@gnu.ac.kr
Received: 2016-09-07
Accepted: 2017-04-21
Abstract
AIM: To
investigate the retinal toxicity and pharmacokinetics of simvastatin
intravitreally injected into mice.
METHODS: Forty-eight
6-8-week-old C57BL/6J mice were used in this study. Simvastatin was
intravitreally injected into the right eye of each mouse; the left eye was
injected with vehicle and was used as a control. Bilateral dark-adapted
electroretinography (ERG) was performed 1 and 7d following injection. Histology
was examined using a combination of light, fluorescence and electron
microscopy. High-performance liquid chromatography (HPLC) was used to determine
the decay in the retinal simvastatin concentration.
RESULTS: ERG
revealed no significant changes in the simvastatin-injected eyes compared to
control. Histologic studies showed normal retinal morphology in eyes injected with
simvastatin up to a final vitreal concentration of 200 μmol/L.
No significant changes in the number of photoreceptors, bipolar cells or
ganglion cells were found. The retinal simvastatin concentration decayed exponentially,
with a half-life of 1.92-2.41h.
CONCLUSION: Intravitreal
injection of up to 200 μmol/L
simvastatin produced no signs of adverse effects in the mouse retina.
Simvastatin reaches the retina shortly after intravitreal injectionand has a
short half-life.
KEYWORDS:
simvastatin; retina; electroretinography; high-performance liquid
chromatography; electron microscopy; intravitreal injection
Citation: Tse DY, Kim SJ, Chung I, He F, Wensel TG, Wu SM. The ocular toxicity and
pharmacokinetics of simvastatin following intravitreal injection in mice. Int
J Ophthalmol
2017;10(9):1361-1369
INTRODUCTION
Statins, potent inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A
(HMG-CoA) reductase, have widely been prescribed for the treatment of
hyperlipidemia since 1987[1]
and have recently been demonstrated to possess an expanding spectrum of
activities, including anti-inflammatory, anti-angiogenic, anti-oxidant[2], immunosuppressive[3] and neuroprotective[4-6] effects. Statins have been
reported to be beneficial to patients with a variety of ocular diseases such as
diabetic retinopathy[7],
age-related macular degeneration (AMD)[8] and retinal ischemia and to potentially be useful in
glaucoma[9-12].
Simvastatin, the semisynthetic statin tested in the present study, is
one of the most potent of all the identified statins; simvastatin inhibits the
diabetes-induced increases in vascular endothelial growth factor (VEGF)
expression, protects the blood-retinal barrier and suppresses the progression
of streptozotocin-induced diabetic retinopathy in rats[13-15]. A recent study has shown that simvastatin activated
the anti-oxidative defense protein HO-1 in cultured human retinal pigment
epithelial (RPE) cells[16]
and that this activity contributed to its cytoprotective effect against AMD.
Considering the potential adverse side effects of intravitreally-injected
steroids [e.g. intraocular pressure (IOP) elevation, cataract] and
anti-VEGF drugs (e.g. intolerance, cost, and unknown deleterious
cumulative systemic effects) commonly used in the treatment in AMD or diabetic
retinopathy, simvastatin might be a potential alternative drug candidate as
several reports have suggested that simvastatin inhibits the progression of
these two diseases[17-20]. It has also been
shown that simvastatin enhanced retinal ganglion cell (RGC) survival and
protected visual functions in rodent models of acute retinal ischemia/reperfusion
injury[21-23] and optic nerve
lesioning[24].
Simvastatin inhibits the Rho/Rho-kinase pathway and thus might have therapeutic
potential in the prevention of cicatrical contraction of proliferative
membranes in vivo study. And thus, simvastatin might provide a new
strategy for the treatment and prevention of the development of proliferative
vitreoretinal diseases[25].
In most of the literature, statins were administered orally or injected
intraperitoneally. From a clinical perspective, some ocular conditions would be
better managed if the intraocular statin concentration was increased more
efficiently via direct intravitreal injection; such conditions include
when ocular conditions are acute or when systemic side effects should be
avoided[26-27]. From a research
perspective, intravitreal injection is superior to systemic delivery routes
because the other eye can serve as an internal control in certain experimental
settings[28].
Our objective was to study the retinal toxicity of simvastatin to mice
using electroretinography (ERG) and electron microscopy. These results are
pivotal in guiding future laboratory and clinical studies on the dosage and
delivery of simvastatin. This study is particularly relevant to mouse research
models, as such disease models are widely available and are easily combined
with genetic manipulations or surgical interventions[29]. The long-term goal of this line
of investigation is to utilize mouse models to investigate the potential
neuroprotective effects of simvastatin against avariety of retinal diseases such
as glaucoma and AMD[30-32].
MATERIALS AND METHODS
Animal Preparations Forty-eight 6-8 weeks old C57BL/6J mice obtained from the Jackson
Laboratory (Bar Harbor, ME, USA) were used for the experiments. The animals
were treated in accordance with the ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research and with the animal welfare guidelines of the
IACUC of Baylor College of Medicine.
Intravitreal Injection Because vitreal simvastatin concentrations of 5 and 15 μmol/L have been shown to be effective and did not produce sign of
toxicity in previous animal studies[24-25],
we selected higher vitreal simvastatin concentrations of 50 and 200 μmol/L for this retinal toxicity study to provide at least 10× margin
over the previous studies.
Simvastatin (#S6196, Sigma-Aldrich Corp. St. Louis, MO, USA) was
converted to its active form according to the manufacturer’s instructions[33], diluted in
sterile buffered balanced salt solutions (pH 7.5; Alcon Laboratories, Inc.,
Fort Worth, TX, USA), and then filtered using a 0.22-μm PVDF filter (Millipore, Billerica, MA, USA). Aliquots were stored at -20℃ before use. The mice were first anesthetized by intraperitoneally injecting weight-based doses of
ketamine (95 mg/mL) and xylazine (5 mg/mL). Then, a single drop of 0.5%
proparacaine hydrochloride (Alcon Laboratories, Inc., Fort Worth, TX, USA) and
1% tropicamide were applied to both eyes. One microliter of 0.5 or 2.0 mmol/L
simvastatin was injected into the vitreous of the right eye under a
stereomicroscope using a Nanofil syringe fitted with a 33-gauge beveled needle
(WPI, Sarasota, FL, USA). One microliter of an equivalent vehicle solution was
injected into the left eye as a control. Assuming that the injected 1 μL of solution was diluted into the 10 μL of vitreous fluid in the eye[34-35],
the vitreal concentrations of simvastatin were 50 and 200 μmol/L for the injected 0.5 and 2.0 mmol/L solutions, respectively. The
needle was inserted behind the limbus through the pars plana at an oblique
angle to avoid damaging the crystallized lens. To prevent the injected solution
from escaping the eye when the needle was withdrawn, the needle tip was held in
the eye for 30s after the injection to facilitate mixing.
Electroretinography Recordings
In vivo scotopic ERG was recorded bilaterally from the mice 1 and 7d after intravitreal
injection. A pre-injection measurement was performed on a different cohort of
mice as a reference. Prior to each ERG assessment, the mice were allowed to
adapt to the dark for at least 2.5h. Under dim redlight, the mice were prepared
for ERG testing as described previously[30]. The signals were amplified
using a Grass P122 amplifier and were band-pass filtered from 0.1 to 1000 Hz
(Grass Instruments, West Warwick, RI, USA). The data were acquired using a
National Instruments data acquisition board (USB-6216, National Instruments,
TX, USA) at a sampling rate of 10 kHz. The traces were analyzed using custom
codes written in MATLAB (MathWorks, Natick, MA, USA)[30].
The flashes used for scotopic b-wave measurements were generated using
cyan light-emitting diodes calibrated with a photometer (ILT1700, International
Light, MA, USA) and were converted to the unit photoisomerizations/rod, where 1
scot cd m2=581 photoisomerizations/rod/s[30]. To remove oscillatory
potentials before fitting, the scotopic b-wave was digitally filtered using the
filtfilt function in MATLAB (low-pass filter; Fc=60 Hz). The positive and
negative scotopic threshold responses (STRs) were measured under stimuli of
various intensities as described previously[30]. The cone ERG recording was
performed according to a paired-flash method using xenon flashes[36]. An initial
conditioning flash saturated both rods and cones 2s before a probe flash. The
ERG signal recorded by the probe flash is attributed to responses driven by the
cones because cones recover faster than rods. In this manuscript, the stimulus
intensities are presented on a log scale, where 1E+0=1 photoisomerizations/rod.
All statistical analyses were performed using paired t-tests in SPSS
version 20 (IBM).
Pharmacokinetic Analysis via High-performance Liquid
Chromatography
The ocular concentration of simvastatin in the current study was
measured using the retinal tissue instead of the vitreous humor, which is
commonly used in rabbit pharmacokinetic models[37-39]. This experimental approach is advantageous because the
retina itself is typically the target of treatment.
The injected eyes were enucleated at 1, 3, 6, 12, 24 or 48h to measure
the retinal concentration of the drug. For each of the above time points, at
least three microcentrifuge tube samples were analyzed. Each sample contained
four whole retinae extracted from four eyes. First, 100 μL of phosphate buffered saline (PBS) at 4℃ was added to the sample before the samples were homogenized using amotorized mortar
(#47747-370, VWR International LLC, Radnor, PA, USA) for a total of 6min. Then,
900 μL of acetonitrile at 4℃ was added to the sample.
The mixture was subsequently vortexed for 2min and then
ultra-centrifuged at 68 000× g for 20min at 4℃. The supernatant was transferred to auto-sampler
vials and then concentrated to a volume of 50-80 µL
using a SpeedVac. The resulting samples contained 50% acetonitrile and 0.1%
(v/v) trifluoroacetic acid (TFA).
The simvastatin standards (Sigma-Aldrich Corp., St. Louis, MO, USA) were
dissolved in 100 μL of 50% acetonitrile containing 0.1%
(v/v) TFA and were vortexed for 1min. All samples were filtered through a 0.45 μm Acrodisc® 3 CR PTFE filter to remove insoluble particles
before injection into a C18 high-performance liquid chromatography (HPLC)
column (Grace Davison Discovery Sciences, 4.6×250 mm, 5-micron, Vydac 218TP
C18).
The samples were analyzed using Shimadzu gradient HPLC (Shimadzu Co.,
Kyoto, Japan) in a system of 0.1% aqueous TFA (buffer A) versus 0.1% TFA in acetonitrile
(buffer B) at a flow rate of 1 mL/min. A 100 μL volume of each sample was injected into the C18 column, which was
pre-equilibrated with 50% buffer B. Simvastatin was eluted with a two-step
gradient of acetonitrile: 50% acetonitrile containing 0.1% TFA for 10min
followed by 80% acetonitrile containing 0.1% TFA for an additional 10min.
Simvastatin was monitored by measuring the absorbance at 238 nm using a
Shimadzu SPD-M20A UV/VIS photodiode array detector (Shimadzu Co.) interfaced to
a computer running Shimadzu LCsolution software. The standard curve, which was
created using eight different concentrations of the simvastatin standard, was
linear from 2 to 640 pmol (correlation coefficient 0.99988). The detection
limit using this method was estimated to be approximately 1.5 pmol
(signal-to-noise ratio greater than 2).
Pharmacokinetic data were then analyzed using Phoenix WinNonlin software
version 5.3 (Certera, St. Louis, MO, USA). The following equation was used for
the one-compartment model: ![]()
where C(t) denotes the quantity of simvastatin at time t, Vd
denotes the volume of distribution, and K(h-1) denotes the
elimination rate constant. In addition, data were analyzed using a non-compartmental
model for reference.
Retinal Histology On the 7th day following injection, anesthetized mice were
euthanatized via cervical dislocation, and their eyes were enucleated
after ERG recording was conducted. For histologic studies, a large full-thickness
incision was made in the cornea, and the anterior segment was removed. Then,
the eye cups were fixed in 3% glutaraldehyde in phosphate buffer. The tissue
was then washed in 1 mol/L sodium phosphate buffer, pH 7.3, and post-fixed in
1% osmium tetroxide for 1h at room temperature. The dehydrated tissue was
infiltrated with acetone and Poly/Bed 812 plastic resin. The tissue was then
embedded in plastic block molds with 100% Poly/Bed 812. Sections (1 μm thick) were generated on an ultramicrotome, placed on glass slides and
stained with toluidine blue for light microscopy. The areas of interest were
trimmed, and 80-nm-thick ultra-thin sections were sliced using a Leica Ultracut
R Ultramicrotome, mounted on 100-mesh copper grids, and stained with 2% uranyl
acetate and Reynold’s lead citrate. The specimens were imaged using a Zeiss CEM
902 electron microscope to study the retinal ultrastructure. For
immunohistochemistry (IHC) and cell counting studies, the eyes were carefully
dissected to isolate whole retinae, which were then incubated in 4%
paraformaldehyde (Electron Microscopy Science, Fort Washington, PA, USA) in
Dulbecco's phosphate-buffered saline (DPBS, pH7.4, Invitrogen, LaJolla, CA,
USA) at room temperature for 45min for fixation. An indirect antibody method
was adopted for IHC. First, retinae were sliced into vertical 40-μm-thick sections using a microtome (Vibratome; Leica Microsystems,
Bannockburn, IL, USA) and then blocked with 10% donkey serum (Jackson
ImmunoResearch, West Grove, PA, USA) in TBS [DPBS containing 0.5% Triton X-100 (Sigma)
and 0.1% sodium azide (Sigma), pH 7.2] at 4℃ overnight to reduce nonspecific
labeling. The free-floating sections were incubated in primary antibodies
diluted in TBS containing 3% donkey serum at 4℃ for 4d. Controls lacking primary antibodies were also processed. After several rinses,
the sections were transferred and incubated in a TBS solution containing 3%
normal donkey serum and donkey-hosted secondary antibodies conjugated with Cy3
(1:200, Jackson ImmunoResearch) or Alexa Fluor 488 (1:200 dilution, Molecular
Probes, Eugene, OR) at 4℃ overnight. A fluorescent
nuclear dye, TO-PRO3 (1:3000 dilution, Molecular
Probes, Eugene, OR, Cat. No. T3605), was applied together with the secondary
antibodies.
After rinsing several times, the sections were mounted with Vectashield
medium (Vector Laboratories, Burlingame, CA, USA), cover slipped and, finally,
observed under a confocal laser scanning microscope (LSM 510; Zeiss, Thornwood,
NY, USA). Images were acquired using Zeiss LSM software and a 40× or a 63×
oil-immersion objective. Adobe Photoshop CS5 (Adobe Systems, San Jose, CA, USA)
was used to crop images and to apply uniform brightness and contrast
adjustments.
In the present study, rod bipolar cells were immuno-labeled with a mouse
antibody against PKCα (1:250 dilution; BD Transduction Labs,
San Jose, CA, USA, Cat. No. 610107)[40]. Photoreceptor nuclei in the outer retina and ganglion
cell nuclei in the inner retina were stained using the fluorescent nuclear dye
TO-PRO3[41].
Cone cell bodies were immuno-labeled with a rabbit antibody against GNAT2
(1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA, Cat. No. SC-390)[42-43]. The dimensions were measured
using built-in tools in Zeiss LSM software.
To determine whether injection of 200 μmol/L simvastatin produced any toxicity to the retina and caused any
retinal cell loss, the thicknesses of the two retinal nuclear layers were
gauged, and the numbers of cone soma, rod bipolar cells and ganglion cells were
systematically counted in the two groups. Five mice, each of which received
injections of 200 μmol/L simvastatin into one eye and vehicle
solution into the other eye, were sacrificed. Three sections from each eye were
imaged, for a sample size of 15 per group; the number of cells in each 200-μm region were counted, and the dimensions were measured.
RESULTS
Electroretinography Changes in the amplitudes of the scotopic a-wave and b-wave against an
increasing stimulus intensity measured 1 and 7d after injection of 50 or 200 μmol/L simvastatin are shown in Figure 1. As shown in panels A-D, eyes
injected with either 50 or 200 μmol/L simvastatin showed no significant
differences in the a- or b-waves at 9 stimulus intensities 1 or 7d after
injection on ERG compared to the control eyes. Baseline a-wave and b-wave
responses from another 7 pairs of eyes before injection are shown in panel E
for reference.
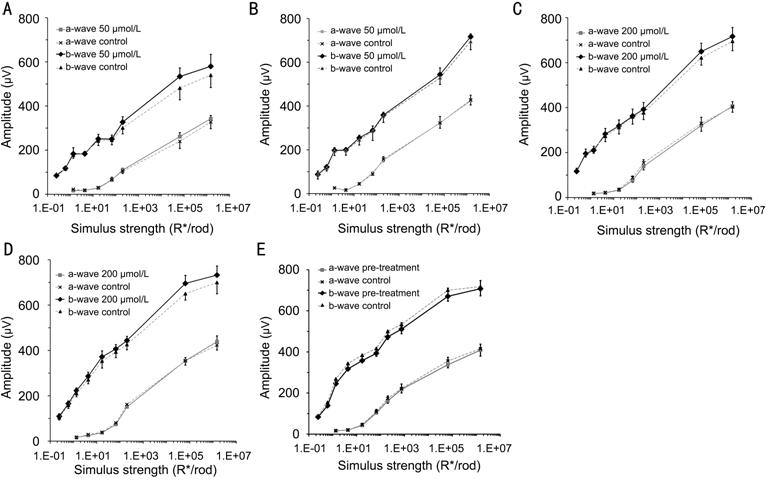
Figure 1 Changes in the amplitudes of the scotopic a-wave and b-wave against
an increasing stimulus intensity measured after injection of simvastatin A: Day 1 after injection of 50 μmol/L
simvastatin; B: Day 7 after injection of 50 μmol/L
simvastatin; C: Day 1 after injection of 200 μmol/L simvastatin; D: Day 7 after injection of 200 μmol/L simvastatin; E: Pre-injection.
To test whether simvastatin produced any toxicity to the retinal cone
pathway, we employed paired flash ERG recordings to isolate the cone-driven
responses from the rod-driven responses. As shown in Figure 2, there were no
significant differences in the cone a-wave or b-wave between the
simvastatin-injected eyes and the vehicle-injected control eyes based on ERG
recordings performed 1 and 7d after injection (7 pairs of eyes). Figure 3 shows
the changes in the positive STRs (pSTRs) and the negative STRs (nSTRs) in
response to increasing stimulus intensities 1 and 7d after injection of 50 or
200 μmol/L simvastatin. There were no significant differences in the mean
pSTR or nSTR between simvastatin-injected eyes and control eyes for either
concentration at each time point. Given that the pSTR and the nSTR represent
inner retinal signaling from third-order neurons such as RGCs and AII amacrine
cells[44-46], our results
suggest that a vitreal simvastatin concentration of 200 μmol/L or less does not alter the electrophysiological functions of
ganglion cells.
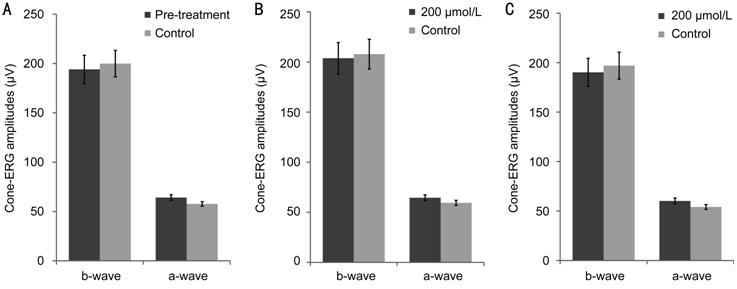
Figure 2 The differences in the cone a-wave or b-wave between the
simvastatin-injected eyes and the vehicle-injected control eyes based on ERG
recordings A: Pre-injection; B: Day 1 after injection of 200 μmol/L simvastatin; C: Day 7 after injection of 200 μmol/L simvastatin.
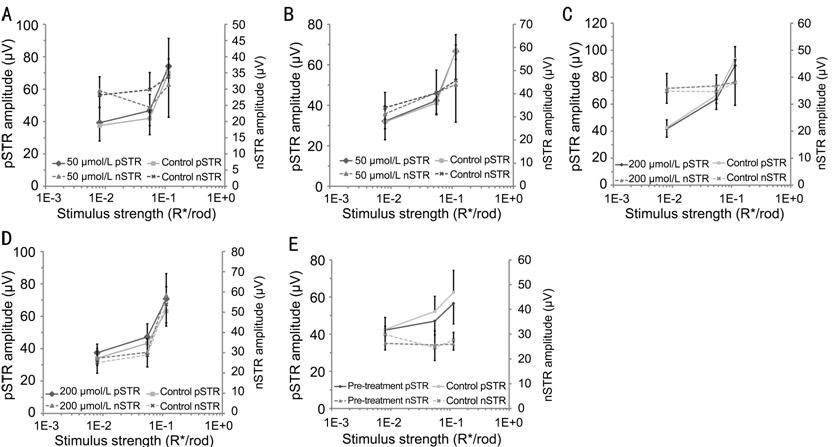
Figure 3 Changes in the
positive STRs (pSTRs) and the negative STRs (nSTRs) in response to increasing
stimulus intensities after injection of simvastatin A: Day 1 after injection of 50 μmol/L
simvastatin; B: Day 7 after injection of 50 μmol/L
simvastatin; C: Day 1 after injection of 200 μmol/L simvastatin; D: Day 7 after injection of 200 μmol/L simvastatin; E: Pre-injection.
Pharmacokinetic Analysis The peak simvastatin concentration was calculated according to
appropriate standard curves using LCsolution software. These standard curves,
created using eight different concentrations of simvastatin, were linear from 0
to 640 pmol (correlation coefficient >0.99988). The detection limit was
below 2 pmol.
Simvastatin was detectable in retinal samples collected 1h following the
injection. Samples collected at the subsequent post-injection time points
showed decreasing concentrations of simvastatin. These findings suggest that
intravitreally injected simvastatin arrives at the retina shortly after
injection; additionally, simvastatin reached its maximum concentration within
1h. The change in the mean simvastatin concentration in the retina over time as
measured using HPLC was fitted using a one-compartment model (Figure 4). The
half-lives of simvastatin for injected two concentrations of 200 and 50 µmol/L
were found to be 1.92 and 2.41h, respectively (Table 1). Projected Cmax
were 506.37 and 75.23 mol, respectively. Mean residence time were 2.77 and
3.48h, respectively.
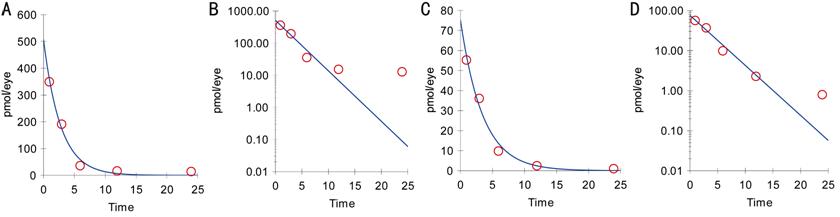
Figure 4 The change in the
mean simvastatin concentration in the retina over time as measured using HPLC
was fitted using a one-compartment model
A, B: Injection of 200 μmol/L simvastatin; C, D: Injection of 50 μmol/L simvastatin.
Table 1 Pharmacokinetic parameters of simvastatin in retina
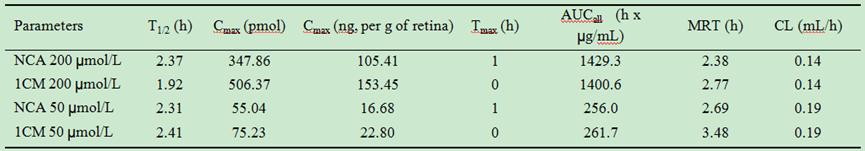
NCA: Non-compartmental analysis; 1CM: 1-compartment modeling; Cmax:
Observed/predicted max quantity; Tmax: Time to Cmax; AUC:
Area under the curve; MRT: Mean residence time; CL: Clearance. Mean wet weight
of mouse retina=3.3 mg; data taken from Cerani et al[47].
Retinal Histology Gross examination of eye specimens revealed no evidence of retinal
tearing, retinal detachment, or retinal hemorrhage or any signs of infection in
any of the simvastatin-injected or control eyes. The mean thicknesses of the
outer nuclear layer (ONL) were 62.5±0.9 and 63.0±1.3 μm in the simvastatin and control groups. The ONL thicknesses in terms of
cell number (rods and cones) were 11.7±0.2 and 11.9±0.2 in the simvastatin and
control groups. The mean numbers of cone soma were 14.5±1.7 and 15.4±1.0 per
200-μm horizontal ONL section in the simvastatin and control groups. Similarly,
the mean numbers of rod bipolar cells (RBCs) in the inner nuclear layer (INL)
were 22.9±0.7 and 22.7±0.5 per 200 μm in the
simvastatin and control groups. The mean thicknesses of the INL were 43.4±1.3
and 44.0±1.8 μm in the simvastatin and control groups. Finally, the numbers of
ganglion cells per 200-μm horizontal ganglion cell layer section
were 23±0.6 and 23.1±0.4 in the simvastatin and control groups (Figure 5).
There was no significant difference (paired t-test, P>0.05) in
the measured layer thicknesses or in the numbers of counted neurons between the
200 μmol/L simvastatin-injected group and the control group (Table 2). These
findings suggested that intravitreally injecting 200 μmol/L simvastatin did not induce a loss of retinal neurons.
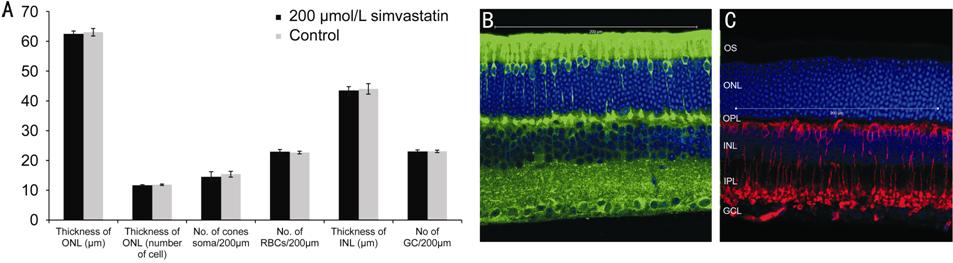
Figure 5 The cellular profiles of the Simvastatin-injected group with
those of the control group A: A bar chart comparing the cellular profiles
of the Simvastatin-injected group with those of the control group. Data points
and error bars represent the mean values and standard errors, respectively. B:
A representative fluorescent micrograph of a retina processed by staining with
the nuclear dye TO-PRO3 (blue) and the antibody GNAT2 (green). A
higher-than-normal exposure was used for the green channel to optimally show
the cone soma and to facilitate cell counting. Ganglion cell soma were stained
in blue in the inner retina. C: A representative fluorescent micrograph of a
retina processed by staining with the antibody PKCα (red) and the nuclear dye TO-PRO3 (blue).
Table 2 Comparing the retinal cellular
profile of simvastatin injected eyes and vehicle injected control eyes

Low magnification histologic examination via light microscopy
conducted 7d after injection revealed normal neurosensory retinae. No signs of
inflammation- or toxicity-induced changes in any retinal layer were found.
There were no observable differences in layer thicknesses or cell morphologies
between the simvastatin-injected retinae and the control retinae (Figure 6).
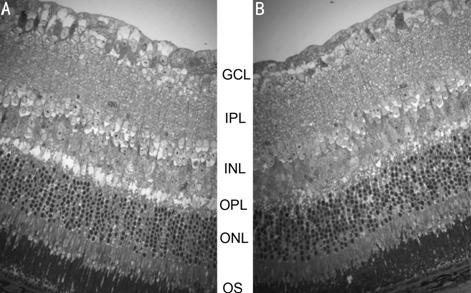
Figure 6 Light micrographs of vertical retinal sections of mouse eyes
processed 7d after intravitreal injection of (A) the control vehicle solution
or (B) 2.0 mmol/L Simvastatin GCL: Ganglion cell layer; IPL: Inner
plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL:
Outer nuclear layer; OS: Outer segment of photoreceptors.
High magnification histologic examination of individual retinal layers via
electron microscopy revealed very similar ultrastructure and cell morphologies
between the simvastatin-injected retinae and the control retinae (Figure 7). No
abnormalities in photoreceptors, bipolar cells or ganglion cells were
identified in the simvastatin-injected eyes 7d after injection.
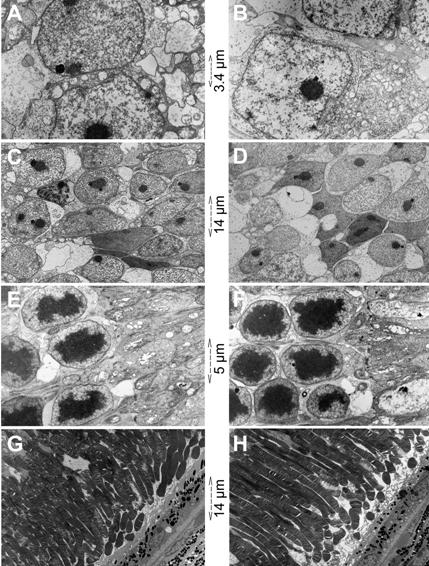
Figure 7 Electron micrographs of vertical retinal sections of mouse eyes
processed 7d after intravitreal injection of (left column) the control vehicle
solution or (right column) 200 μmol/L Simvastatin A, B: Ganglion cells; C, D: Inner nuclear layer; E, F: Photoreceptor
inner segment; G, H: Photoreceptor outer segment and retinal pigment
epithelium.
DISCUSSION
Simvastatin has been suggested to have a pleiotropic protective effect
against a variety of ocular diseases, including diabetic retinopathy, AMD,
retinal ischemia and glaucoma. More recently, its protective effect against
glaucomatous neuronal damage has been hypothesized to be related to its
anti-inflammatory properties[11].
However, the ocular toxicity and pharmacokinetics of simvastatin had not been
adequately studied previously. In the present study, we have shown that
intravitreal injection of up to 200 μmol/L
simvastatin produced no apparent adverse effects in the mouse retina based on
results from functional tests via ERG and histologic examinations via
fluorescence and electron microscopy.
ERG is a useful tool for evaluating retinal functions both
experimentally and clinically. In the present study, ERG was performed using a
comprehensive range of stimuli to assess the functions of a comprehensive range
of retinal neurons. Depending on the stimulus intensity, the a-wave and the
b-wave are generated via the rod pathway, the cone pathway or a
combination of the two. The scotopic a-wave and b-wave data suggest that
simvastatin up to a vitreal concentration of 200 μmol/L does not influence the functions of rod photoreceptors or
rod-driven bipolar cells. There were no significant differences in the cone
a-wave or b-wave between the simvastatin-injected eyes and the control eyes.
This observation indicated that a vitreal simvastatin concentration of 200 μmol/L did not produce any signs of toxicity to the retinal cone pathway.
The pSTR and the nSTR are the most sensitive components of the ERG, and our
results suggest that a vitreal simvastatin concentration of 200 μmol/L or less does not alter the electrophysiological functions of
ganglion cells.
Traditionally, statins are administered systemically via oral
ingestion, subcutaneous injection, or intraperitoneal injection. In contrast,
delivery via intravitreal injection has not been commonly used but has
the advantage of enabling the application of a higher dose intraocularly and
monocularly within a short interval. Intravitreal injection is also likely to
be more efficient when the therapeutic target site is located at the proximal
retina, where drugs administered systemically moving from the choroid must
overcome diffusion barriers to reach the retina. One successful application of
simvastatin was that intravitreally administered 15 μmol/L simvastatin had no apparent adverse effects in rabbits and
prevented the progression of induced proliferative vitreoretinopathy[25].
We found that the ocular half-life of simvastatin in the retina
following intravitreal administration was 1.92-2.41h; this half-life is
relatively short compared with that of other drugs administered via
intravitreal injection. For example, the half-lives of dexamethasone and
triamcinolone were previously reported to be 3.48h and 1.57d, respectively[37-38]. We cannot directly compare
those findings to the present results because the previous studies performed
measurements on the rabbit eye. We did not find any related data using mice. In
the present study, the HPLC data suggested that intravitreally injected
simvastatin rapidly reached the retina and had a short retinal half-life. Our
findings indicate that a sustained release mechanism such as an intravitreal
implant[48]
would be needed to extend the application of simvastatin to the treatment of
chronic vitreal-retinal diseases.
Since the calculated half-life is about 2h and simvastatin is mostly
cleared in 12h, the 1d time point is appropriate to evaluate acute toxicity and
the 1wk time point was designed to evaluate delayed damages, for example, those
resulted from inflammation or secondary degeneration. Our results have further
shown that administering up to 200 μmol/L
simvastatin via intravitreal injection produced no apparent adverse
effects on the retinal ultrastructure of mouse eyes, as the retinae showed no
signs of anomalies one week after injection. Therefore, a vitreal simvastatin
concentration between 50 and 200 μmol/L
would be a reasonable starting point for future therapeutic trials using mouse
models of acute ocular diseases.
Though the weakest point of this study is that the volume of the mouse
vitreous is much smaller than that of the human vitreous, the present study is
the first to assess the toxicity of simvastatin in mice, which represent a very
useful model animal because of their potential for genetic modification and the
wide availability of mouse models of disease. This study represents an important
step in the exploration of the full potential benefits of simvastatin to
patients with ocular disorders, including diabetic retinopathy, AMD, retinal
ischemia, glaucoma and proliferative vitreoretinopathy. Intravitreal injection
of simvastatin is a highly efficient route of delivery, but the half-life of
intravitreally injected simvastatin is relatively short. To extend its
application to the treatment of chronic ocular disorders, a slow-release drug
delivery system or vehicle might be necessary to sustain a therapeutic
simvastatin dosage in the retina.
ACKNOWLEDGEMENTS
We thank Zhuo Yang for help with animal husbandry and retina extraction,
Ralph Nichols for help with electron microscopy, and Roy Jacoby for carefully
reading the manuscript.
Foundations: Supported by the
National Institute of Health under Award Number R01 EY004446 & R01
EY019908, NIH Vision Core EY02520, the Retina Research Foundation (Houston),
Research to Prevent Blindness Inc., and Hong Kong Polytechnic University grants
G-UA7J and G-YBQT.
Conflicts of Interest: Tse DY, None; Kim SJ, None; Chung I, None; He F, None; Wensel
TG, None; Wu SM, None.
REFERENCES
1 Molgaard J, von Schenck H, Olsson AG.
Effects of simvastatin on plasma lipid, lipoprotein and apolipoprotein
concentrations in hypercholesterolaemia. Eur
Heart J 1988;9(5):541-551. [CrossRef] [PubMed]
2 Cimino M, Gelosa P, Gianella A, Nobili
E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic
brain. Neuroscientist
2007;13(3):208-213. [CrossRef] [PubMed]
3 Kwak B, Mulhaupt F, Veillard N, Pelli G,
Mach F. The HMG-CoA reductase inhibitor simvastatin inhibits IFN-gamma induced
MHC class II expression in human vascular endothelial cells. Swiss Med Wkly 2001;131(3-4):41-46. [PubMed]
4 Zacco A, Togo J, Spence K, Ellis A,
Lloyd D, Furlong S, Piser T. 3-hydroxy-3-methylglutaryl coenzyme A reductase
inhibitors protect cortical neurons from excitotoxicity. J Neurosci 2003;23(35):11104-11111. [PubMed]
5 Vaughan CJ, Delanty N. Neuroprotective
properties of statins in cerebral ischemia and stroke. Stroke 1999;30(9):1969-1973. [CrossRef] [PubMed]
6 Menge T, von Büdingen HC, Zamvil SS,
Hartung HP, Kieseier BC, Stüve O. Statins for treatment of CNS diseases. Status
report from research and clinical practice. Nervenarzt
2005;76(4):426-437. [CrossRef] [PubMed]
7 Gordon B, Chang S, Kavanagh M, Berrocal
M, Yannuzzi L, Robertson C, Drexler A. The effects of lipid lowering on
diabetic retinopathy. Am J Ophthalmol
1991;112(4):385-391. [CrossRef]
8 Tan JSL, Mitchell P, Rochtchina E, Wang
JJ. Statins and the long-term risk of incident age-related macular
degeneration: the blue mountains eye study. Am
J Ophthalmol 2007;143(4):685-687. [CrossRef] [PubMed]
9 Stein JD, Newman-Casey PA, Talwar N, Nan
B, Richards JE, Musch DC. The relationship between statin use and open-angle
glaucoma. Ophthalmology
2012;119(10):2074-2081. [CrossRef] [PMC free article] [PubMed]
10 Leung DY, Li FC, Kwong YY, Tham CC, Chi
SC, Lam DS. Simvastatin and disease stabilization in normal tension glaucoma: a
cohort study. Ophthalmology
2010;117(3):471-476. [CrossRef] [PubMed]
11 Soto I, Howell GR. The complex role of
neuroinflammation in glaucoma. Cold
Spring Harb Perspect Med 2014;4(8). pii:017269. [CrossRef] [PMC free article] [PubMed]
12 Davaro F, Forde SD, Garfield M, Jiang
Z, Halmen K, Tamburro ND, Kurt-Jones E, Fitzgerald KA, Golenbock DT, Wang D. 3-Hydroxyl-3-methylglutaryl coenzyme A
(HMG-CoA) reductase inhibitor (statin)-induced 28-kDa interleukin-1beta
interferes with mature IL-1beta signaling. J
Biol Chem 2014;289(23):16214-16222. [CrossRef] [PMC free article] [PubMed]
13 Miyahara S, Kiryu J, Yamashiro K,
Miyamoto K, Hirose F, Tamura H, Katsuta H, Nishijima K, Tsujikawa A, Honda Y. Simvastatin inhibits leukocyte
accumulation and vascular permeability in the retinas of rats with
streptozotocin-induced diabetes. Am J
Pathol 2004;164(5):1697-1706. [CrossRef]
14 Zhang W, Yan H. Simvastatin increases
circulating endothelial progenitor cells and reduces the formation and
progression of diabetic retinopathy in rats. Exp Eye Res 2012;105:1-8. [CrossRef] [PubMed]
15 Al-Shabrawey M, Bartoli M, El-Remessy
AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH oxidase and Stat3 in statin-mediated protection
against diabetic retinopathy. Invest
Ophthalmol Vis Sci 2008;49(7):3231-3238. [CrossRef] [PMC free article] [PubMed]
16 Kim KJ, Kim KS, Kim NR, Chin HS.
Effects of simvastatin on the expression of heme oxygenase-1 in human RPE
cells. Invest Ophthalmol Vis Sci
2012;53(10):6456-6464. [CrossRef] [PubMed]
17 James ER. The etiology of steroid
cataract. J Ocul Pharmacol Ther 2007;23(5):403-420.
[CrossRef] [PubMed]
18 Binder S. Loss of reactivity in
intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol 2012;96(1):1-2. [CrossRef] [PubMed]
19 Dibas A, Yorio T. Glucocorticoid
therapy and ocular hypertension. Eur J
Pharmacol 2016;787:57-71. [CrossRef] [PubMed]
20 Scott AW, Bressler SB. Long-term
follow-up of vascular endothelial growth factor inhibitor therapy for
neovascular age-related macular degeneration. Curr Opin Ophthalmol 2013;24(3):190-196. [CrossRef] [PubMed]
21 Schmeer C, Gamez A, Tausch S, Witte OW,
Isenmann S. Statins modulate heat shock protein expression and enhance retinal
ganglion cell survival after transient retinal ischemia/reperfusion in vivo. Invest Ophthalmol Vis Sci
2008;49(11):4971-4981. [CrossRef] [PubMed]
22 Krempler K, Schmeer CW, Isenmann S,
Witte OW, Löwel S. Simvastatin improves retinal ganglion cell survival and
spatial vision after acute retinal ischemia/reperfusion in mice. Invest Ophthalmol Vis Sci
2011;52(5):2606-2618. [CrossRef] [PubMed]
23 Ko ML, Chen CF, Peng PH, Peng YH.
Simvastatin upregulates Bcl-2 expression and protects retinal neurons from
early ischemia/reperfusion injury in the rat retina. Exp Eye Res 2011;93(5):580-585. [CrossRef] [PubMed]
24 Kretz A, Schmeer C, Tausch S, Isenmann
S. Simvastatin promotes heat shock protein 27 expression and Akt activation in
the rat retina and protects axotomized retinal ganglion cells in vivo. Neurobiol Dis 2006;21(2):421-430. [CrossRef] [PubMed]
25 Kawahara S, Hata Y, Kita T, Arita R,
Miura M, Nakao S, Mochizuki Y, Enaida H, Kagimoto T, Goto Y, Hafezi-Moghadam A,
Ishibashi T. Potent inhibition of cicatricial contraction in proliferative
vitreoretinal diseases by statins. Diabetes
2008;57(10):2784-2793. [CrossRef] [PMC free article] [PubMed]
26 Sasaki H, Yamamura K, Mukai T, Nishida
K, Nakamura J, Nakashima M, Ichikawa M.
Enhancement of ocular drug penetration. Crit
Rev Ther Drug Carrier Syst 1999;16(1):85-146. [CrossRef]
27 Lee JE, Lim DW, Park HJ, Shin JH, Lee
SM, Oum BS. Intraocular toxicity and pharmacokinetics of candesartan in a
rabbit model. Invest Ophthalmol Vis Sci
2011;52(6):2924-2929. [CrossRef] [PubMed]
28 Chiu K, Chang RC, So KF. Intravitreous
injection for establishing ocular diseases model. J Vis Exp 2007;8:313. [CrossRef]
29 Levkovitch-Verbin H. Animal models of
optic nerve diseases. Eye (Lond)
2004;18(11):1066-1074. [CrossRef] [PubMed]
30 Frankfort BJ, Khan AK, Tse DY, Chung I,
Pang JJ, Yang Z, Gross RL, Wu SM.
Elevated intraocular pressure causes inner retinal dysfunction before cell loss
in a mouse model of experimental glaucoma. Invest
Ophthalmol Vis Sci 2013;54(1):762-770. [CrossRef] [PMC free article] [PubMed]
31 Reichstein D, Ren L, Filippopoulos T,
Mittag T, Danias J. Apoptotic retinal ganglion cell death in the DBA/2 mouse
model of glaucoma. Exp Eye Res
2007;84(1):13-21. [CrossRef] [PubMed]
32 Barathi VA, Yeo SW, Guymer RH, Wong TY,
Luu CD. Effects of simvastatin on retinal structure and function of a high-fat
atherogenic mouse model of thickened Bruch's membrane. Invest Ophthalmol Vis Sci 2014;55(1):460-468. [CrossRef] [PubMed]
33 Sadeghi MM, Collinge M, Pardi R, Bender
JR. Simvastatin modulates cytokine-mediated endothelial cell adhesion molecule
induction: involvement of an inhibitory g protein. J Immunol 2000;165(5):2712-2718. [CrossRef]
34 Remtulla S, Hallett PE. A schematic eye
for the mouse, and comparisons with the rat. Vision Res 1985;25(1):21-31. [CrossRef]
35 Lebrun-Julien F, Bertrand MJ, De Backer
O, Stellwagen D, Morales CR, Di Polo A, Barker PA. ProNGF induces TNFalpha-dependent death of retinal ganglion cells
through a p75NTR non-cell-autonomous signaling pathway. Proc Natl Acad Sci U S A 2010;107(8):3817-3822. [CrossRef] [PMC free article] [PubMed]
36 Pennesi ME, Howes KA, Baehr W, Wu SM.
Guanylate cyclase-activating protein (GCAP) 1 rescues cone recovery kinetics in
GCAP1/GCAP2 knockout mice. Proc Natl Acad
Sci U S A 2003;100(11):6783-6788. [CrossRef] [PMC free article] [PubMed]
37 Abd-El-Barr MM, Albini TA, Carvounis
PE, He F, Manzano RP, Chevez-Barrios P, Wensel TG, Wu SM, Holz ER. Safety and pharmokinetics of
triamcinolone hexacetonide in rabbit eyes. J
Ocul Pharmacol Ther 2008;24(2):197-205. [CrossRef] [PubMed]
38 Kwak HW, D'Amico DJ. Evaluation of the
retinal toxicity and pharmacokinetics of dexamethasone after intravitreal
injection. Arch Ophthalmol
1992;110(2):259-266. [CrossRef]
39 Chin HS, Park TS, Moon YS, Oh JH.
Difference in clearance of intravitreal triamcinolone acetonide between
vitrectomized and nonvitrectomized eyes. Retina
2005;25(5):556-560. [CrossRef]
40 Zhang J, Yang Z, Wu SM. Development of
cholinergic amacrine cells is visual activity-dependent in the postnatal mouse
retina. J Comp Neurol
2005;484(3):331-343. [CrossRef] [PubMed]
41 Vekslin S, Ben-Yosef T. Spatiotemporal
expression pattern of ceramide kinase-like in the mouse retina. Mol Vis 2010;16:2539-2549. [PMC free article]
[PubMed]
42 Chang B, Dacey MS, Hawes NL, Hitchcock
PF, Milam AH, Atmaca-Sonmez P, Nusinowitz S, Heckenlively JR. Cone photoreceptor function loss-3, a
novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci 2006;47(11):5017-5021.
[CrossRef] [PubMed]
43 Kostic C, Crippa SV, Pignat V,
Bemelmans AP, Samardzija M, Grimm C, Wenzel A, Arsenijevic Y. Gene therapy regenerates protein
expression in cone photoreceptors in Rpe65(R91W/R91W) mice. PLoS One 2011;6(2):e16588. [CrossRef] [PMC free article] [PubMed]
44 Saszik SM, Robson JG, Frishman LJ. The
scotopic threshold response of the dark-adapted electroretinogram of the mouse.
J Physiol (Lond) 2002;543(Pt
3):899-916. [CrossRef] [PMC free article] [PubMed]
45 Abd-El-Barr MM, Pennesi ME, Saszik SM,
Barrow AJ, Lem J, Bramblett DE, Paul DL, Frishman LJ, Wu SM. Genetic dissection of rod and cone pathways in the dark-adapted
mouse retina. J Neurophysiol
2009;102(3):1945-1955. [CrossRef] [PMC free article] [PubMed]
46 Moshiri A, Gonzalez E, Tagawa K, Maeda
H, Wang M, Frishman LJ, Wang SW. Near
complete loss of retinal ganglion cells in the math5/brn3b double knockout
elicits severe reductions of other cell types during retinal development. Dev Biol 2008;316(2):214-227. [CrossRef] [PMC free article] [PubMed]
47 Cerani A, Tetreault N, Menard C,
Lapalme E, Patel C, Sitaras N, Beaudoin F, Leboeuf D, De Guire V, Binet F,
Dejda A, Rezende FA, Miloudi K, Sapieha P. Neuron-derived semaphorin 3A is an
early inducer of vascular permeability in diabetic retinopathy via
neuropilin-1. Cell Metab
2013;18(4):505-518. [CrossRef] [PubMed]
48 Chang-Lin JE, Attar M, Acheampong AA,
Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release
dexamethasone intravitreal implant. Invest
Ophthalmol Vis Sci 2011;52(1):80-86. [CrossRef] [PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)