IF in JCR CiteScore
Rank About IJO Current
Issue Featured Articles Articles In Press Recent Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1374-1378
·Clinical Research·
Effect of phacoemulsification on intraocular
pressure in patients with primary open angle glaucoma and pseudoexfoliation
glaucoma
Jesus Jimenez-Roman1, Gabriel Lazcano-Gomez1,
Karina Martínez-Baez1, Mauricio Turati1, Rosario
Gulías-Cañizo2,3, Luis F. Hernández-Zimbrón2, Lenin
Ochoa-De la Paz2, Rubén Zamora2, Roberto Gonzalez-Salinas2
1Glaucoma Department, Asociation to Prevent Blindness in Mexico, Mexico
City 04030, Mexico
2Research Department, Asociation to Prevent Blindness in Mexico, Mexico
City 04030, Mexico
3Cell Biology Department, Advanced Research Center, I.P.N. (CINVESTAV) in
Mexico City, Mexico City 07360, Mexico
Correspondence to: Roberto Gonzalez-Salinas. Vicente García Torres 46, Barrio San Lucas,
Coyoacán, Mexico City 04030, Mexico. dr.gonzalezsalinas@apec.com.mx
Received:
2017-01-14
Accepted: 2017-04-14
Abstract
AIM: To
compare the effect of phacoemulsification on intraocular pressure (IOP) in
patients with primary open angle glaucoma (POAG) and pseudoexfoliation glaucoma
(PXG).
METHODS: A
retrospective comparative case series conducted at the Glaucoma Department at
the Association to Prevent Blindness in Mexico. The study enrolled consecutive
patients having phacoemulsification with intraocular lens (IOL)
implantation and a diagnosis of POAG or PXG. Data about IOP values and number
of glaucoma medications used was collected at baseline, 1, 3, 6 and 12mo
postoperatively.
RESULTS: The
study enrolled 88 patients (88 eyes). After phacoemulsification, there was a
statistically significant reduction in IOP values and glaucoma medications use
compared to baseline in both POAG and PXG patients (P<0.001). In the
POAG group, a 20% decrease in IOP values was evidenced, and a 56.5% reduction
in the number of medications used at the one-year follow-up. The PXG group
showed a 20.39%, and a 34.46% decrease in IOP and number of medications used,
respectively. A significant difference in the mean ΔIOP
(postoperative changes in IOP) was evidenced between groups (P=0.005).
The reduction of the postsurgical IOP mean values in both groups, the POAG
group showed a greater reduction in IOP values compared to the PXG group.
CONCLUSION: In
both types of glaucoma, phacoemulsification cataract surgery can result in a
significant IOP reduction (20%) over a 12mo follow-up period. The number of
medications used is also significantly reduced up to 12mo after surgery,
especially in the PXG group.
KEYWORDS: cataract
surgery; pseudoexfoliation glaucoma; secondary glaucoma; primary open angle
glaucoma; intraocular pressure
Citation: Jimenez-Roman J,
Lazcano-Gomez G, Martínez-Baez K, Turati M, Gulías-Cañizo R, Hernández-Zimbron
LF, Ochoa-De la Paz L, Zamora R, Gonzalez-Salinas R. Effect of phacoemulsification on intraocular
pressure in patients with primary open angle glaucoma and pseudoexfoliation
glaucoma. Int J Ophthalmol
2017;10(9):1374-1378
INTRODUCTION
Pseudoexfoliation (PXF) syndrome is a
systemic disorder of unknown etiology with the potential for many intraocular
complications[1]. PXF is an age-related disorder
characterized by the production and accumulation of an abnormal PXF fibrillar
material in various ocular tissues[2]. PXF
material accumulations mechanically weaken the zonular lamellae and impair
zonular anchoring to the ciliary epithelial basement membrane at both its
origin and insertion[3]. In addition, previous
studies have demonstrated that higher cataract grade and shallower preoperative
anterior chamber are key risk factors for endothelial cells reduction after
cataract surgery in eyes with PXF[3-7].
Although the prevalence described varies between series in different countries
and specific populations[1,8],
it has been reported this syndrome affects about 0.2%-30% of people older than
60y worldwide[8].
PXF remains an important risk factor related
to ocular complications during cataract surgery due to its association with
high intraocular pressure (IOP), reduced pupil dilation and zonular weakness[9-10]. Pseudoexfoliation glaucoma (PXG)
is the most common form of secondary open angle glaucoma and develops in the
context of PXF[11-12]. Glaucoma
frequently occurs in eyes with PXF syndrome and compared to primary open angle
glaucoma (POAG), optic damage is more pronounced in these eyes at the time of
diagnosis, and response to medical therapy is poorer[13].
Previous series have demonstrated that
postoperative IOP is directly related with preoperative IOP values[14-15]: the higher the preoperative
IOP, the greater the postoperative IOP reduction[15-16]. In addition, recent studies on controlled POAG
patients have demonstrated a modest decrease in IOP after undergoing
phacoemulsification surgery[17]. However, it has
been suggested that changes in IOP after cataract surgery can be different
among glaucoma types and ethnic groups[17-18].
POAG and PXG are the most common types of chronic open angle glaucoma worldwide[6]; and it has been described that uncomplicated
phacoemulsification with posterior chamber intraocular lens (IOL) implantation surgery alone lowers IOP
and reduces their need for anti-glaucomatous drugs[3-4].
The purpose of this study was to determine
long-term reduction in IOP and glaucoma medications use after routine cataract phacoemulsification surgery in patients
with PXG in comparison to those with POAG.
SUBJECTS AND METHODS
Study Design This retrospective,
observational and comparative study was approved by the Internal Review Board of the Association to Prevent Blindness in Mexico. All the procedures conformed to the tenets of
the Declaration of Helsinki. All participants signed a written informed consent
before surgical procedures were performed.
Patients The medical
records of patients with a diagnosis of POAG or PXG that underwent
phacoemulsification cataract surgery from January 2014 to January 2016 at the Glaucoma Department of the Association to Prevent Blindness in Mexico were analyzed.
Data collected from records included: age,
gender, IOP at all time intervals, and medication used. This analysis comprised
eyes of consecutive patients that had routine phacoemulsification surgery.
Eligibility criteria included: age ≥50y, diagnosis of POAG or exfoliative glaucoma (XFG) in the presence of a cataract that decreased visual acuity and
evidence of glaucomatous optic nerve changes and/or visual field defects related
to glaucoma damage, with an IOP of ≤25 mm Hg. All patients were diagnosed with
glaucoma using functional and/or structural studies. Functional studies
included 24-2 visual fields (Humphrey© Field Analyzer 750i, Carl
Zeiss, Germany). In addition, optical coherence tomography
(Cirrus© HD OCT, Carl Zeiss, Germany) was employed for structural
analysis.
All eyes were examined before surgery,
including a complete slit-lamp evaluation under pharmacological pupil dilation.
Exclusion criteria included: ocular history of any laser procedure or incisional
surgery; history of acute IOP elevation; IOP >25 mm Hg and inability to
complete study procedures.
Two IOP measurements were obtained for each
eye by the same ophthalmologist between 9:00 a.m. and 12:00 a.m. during
preoperative and postoperative visits. From the two IOP measurements, a mean
IOP value was derived for statistical analysis. If the two IOP values differed
by more than 2 mm Hg, the ophthalmologist would
perform a third IOP measurement, and the median value was utilized in the statistical
analysis.
Surgical
Technique A standard
Stop&Chop technique using topical
anesthesia was performed in all cases. Clear corneal incisions of 2.8 mm were
made and manually created capsulorhexes of 5.0 to 5.5 mm were utilized for all
surgeries. The same ophthalmic viscosurgical device (OVD) Duovisc®
(sodium hyaluronate 3%-chondroitin
sulfate 4.0% with sodium hyaluronate 1.0%; Alcon Laboratories, Inc. Fort Worth, Texas, USA) was
utilized in all surgical procedures. Fluid parameters were set as follows: vacuum limit 350, aspiration flow rate 40
mL/min. Ultrasound power was set
according to the lens density of each patient. After cataract removal and
aspiration of cortical material, the appropriate IOL was implanted in the
capsular bag, removing the remaining OVD from the anterior chamber; finalizing
the surgical procedure.
Statistical Analysis Given an α
of 0.05, a β of 0.20, a standard deviation of 1.00, and a power of 0.80, the
estimated study sample size was 43.5 per group. The statistical significance of changes in IOP was determined by a
Wilcoxon match-pairs signed rank test. The comparison among time intervals was
assessed by the Kruskal-Wallis test. In addition, a Dunn multiple comparison
test was used to compare the preoperative IOP measurements with postoperative
time intervals. A P value less than 0.05 was considered statistically
significant. Normal and non-normal distributions were determined by
Shapiro-Wilk tests for all variables. Statistical analyses were performed using
the Statistical Package for Social Sciences (SPSS) software (version 20, SPSS,
Inc., Chicago, IL, USA). Graphs and layouts
depicted in Figures were elaborated using the 2015 GraphPad software Inc. Prism
version 6.0.
RESULTS
A total of 88 patients were enrolled in the study,
44 per group. Clinical and demographic data are summarized on Table 1.

Figure 1 depicts the effect of
phacoemulsification cataract surgery on the mean IOP at each time interval.
There was a statistically significant reduction in IOP compared to preoperative
values at all time intervals from 1 to 12mo postoperatively. In the POAG group,
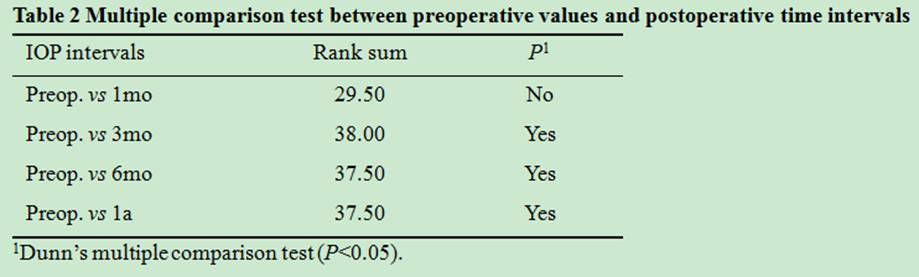
IOP diminished compared to baseline at all time points (Table 2).
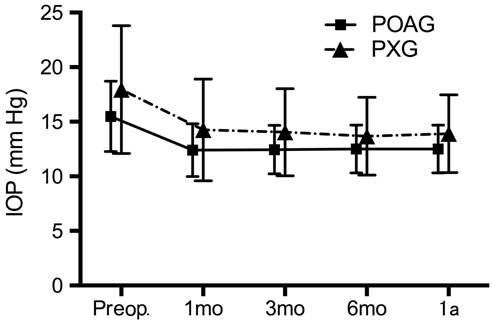
Figure 1 IOP values comparison between groups A statistically significant reduction in mean IOP over preoperative
values at all postoperative time intervals (Kruskal-Wallis test; P<0.0001).
The decrease was significantly greater than
in the PXG group at 3, 6 and 12mo postoperatively. A significant difference in the mean ΔIOP was evidenced between
groups as shown in Table 3.
Table 3 Impact of postoperative IOP on IOP reduction evidenced by ΔIOP for each group
postoperative change in IOP
mm Hg
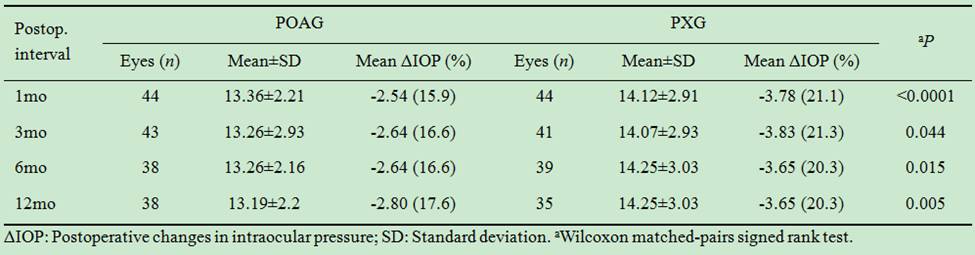
In the PXG group the mean IOP before surgery
was 17.9 mm Hg with a mean of 2.06
medications used, which decreased to a mean of 14.25 mm Hg postoperatively with a mean of 1.35 medications used after 12mo
of follow-up, which represents a 20.3%, and a 34.46% decrease in IOP and number
of medications used, respectively.
The mean IOP before surgery in the POAG group was 15.9 mm Hg with a mean of 2.3 medications used, which decreased to 13.1 mm Hg with a mean of 1.1 medications used during the 12mo follow-up. This represents a 20.0% decrease in
IOP, and a 56.5% reduction in the number of medications used.
Preoperatively, 34 patients in the PXG group
required glaucoma medications. During the 12mo follow-up, the number of
medications used diminished in all patients and also 10 patients discontinued
medication use due to IOP decrease. No patient required additional glaucoma
medications postoperatively.
DISCUSSION
In our study, the mean postoperative IOP at 12mo was significantly lower than the respective preoperative values.
Moreover, the mean ΔIOP difference was also statistically significant between groups
(P<0.0001). This difference suggests that despite the reduction of the postsurgical IOP mean values in
both groups, the POAG group showed
a greater reduction in IOP values compared to the PXG group. Our findings agree
with previous studies that documented an improvement in glaucoma control after phacoemulsification. Mierzejewski et al[19], reported in PXG patients, a decrease in IOP from 20.6 to 15.1 mm Hg (a 27% reduction; P<0.00001). In addition, the number
of medications used decreased from 1.7 to 1.2, similar to our results[12]. Also reported a 5% increase in postoperative IOP, but the glaucoma
severity was not reported and therefore
poorly controlled patients may have minor improvements postoperatively.
Other series have demonstrated a greater IOP
reduction postoperatively in elderly patients, females, eyes with an axial length ≤21 mm, and PXF patients[17-18,20]. However, it has been described that in patients with certain types
of glaucoma, mean IOP may be reduced up to 5.5 mm Hg[16,18]. A recent Meta-analysis evaluated the impact of phacoemulsification
on IOP in glaucoma patients, which reported that in POAG patients who are
controlled with 1 or 2 medications, phacoemulsification alone results in a
modest decrease in IOP (13%) as well as in medication use (12%)[17]. Furthermore,
this analysis reported that incisional glaucoma surgery would be rarely
necessary for IOP control within 1y[17-18]. In
patients with mild to moderate PXG controlled with 1 or 2 medications,
phacoemulsification results in a moderate decrease in IOP (20%) and in the
number of medications required after surgery (35%)[18].
Shingleton et al[21]
studied 240 eyes, also with medically controlled PXG, in patients who underwent
uncomplicated phacoemulsification. The extent of glaucoma damage was not
reported. Among 51 eyes with a follow-up of 60mo, the IOP decreased from 18.0
to 16.9 mm Hg (6%; P<0.030), and
the number of medications used decreased from a mean of 1.6 to 1.0 (38%),
similar to the reduction obtained in the PXG group in our study.
In addition, among studies including PXG and
non-PXG patients, Peräsalo[22], retrospectively studied 182 Finnish patients (226 eyes) with
medically controlled PXG (n=124) and POAG (n=102) who underwent
phacoemulsification cataract surgery. The IOP decreased from 17.1 to15.3 mm Hg (P<0.001) at 12mo of
follow-up. The number of medications used decreased from a mean of 1.5 to 0.9 (40%); but 37% of the patients in the study
required an increase in medications[22]. This
study included PXG and POAG patients, and reported similar reductions both in
IOP and in postoperative medication use; however, no significant differences were evidenced between groups. Similarly, Elguin et al[3]
reported no significant differences in postoperative IOP measurements between
PXG and POAG patients undergoing uneventful cataract surgery.
Several studies have shown that the decrease
in IOP after phacoemulsification is more pronounced in eyes with a higher
preoperative IOP[16]. However, few studies have
evaluated the postoperative IOP response in patients with PXG compared to those
with POAG. It has been suggested that phacoemulsification removes a source of PXF material (the anterior lens capsule) and results in or stimulates
clearance of PXF and pigment
debris from the anterior segment, in particular the trabecular meshwork[1].
Various IOP reduction mechanisms
after phacoemulsification have been proposed, however, the key mechanism may vary across different types of
glaucoma[23]. IOP drop following phacoemulsification has been shown to be
greater in patients with PXF[17]. In addition, it has been described that IOP response after
phacoemulsification surgery in patients with PXF correlated with the volume of
irrigation fluid used intraoperatively, thus reinforcing the idea that the
procedure may remove exfoliation material from the outflow system[17,24].
This study has some limitations that should
be noted, one of the main weaknesses of this study is its retrospective nature
with the inherent limitations of data extrapolation, and therefore subject to the selection bias of such a study. In addition, this study examined IOP alone and did not evaluate
the status of the optic nerve head, nerve fiber layer, or visual fields in the
disease population.
In summary, our findings suggest that
inpatients diagnosed with PXG or POAG, controlled
with 1 or 2 medications and IOP >25 mm Hg, cataract phacoemulsification
surgery results in a significant decrease in IOP, as well as in the number of
medications required after surgery. Therefore, early cataract surgery may be
considered for the treatment of patients with a visually significant cataract
and glaucoma as a reasonable surgical option in patients with coexisting cataract and relatively well-controlled glaucoma.
ACKNOWLEDGEMENTS
We wish to acknowledge the Association to Prevent Blindness in Mexico for the facilities to carry out
this study.
Conflicts of Interest: Jimenez-Roman J, None; Lazcano-Gomez G, None; Martínez-Baez
K, None; Turati M, None; Gulías-Cañizo R, None; Hernández-Zimbrón
LF, None; Ochoa-De la Paz L, None; Zamora R, None; Gonzalez-Salinas
R, None.
REFERENCES
1
Merkur A, Damji KF, Mintsioulis G, Hodge WG. Intraocular pressure decrease
after phacoemulsification in patients with pseudoexfoliation syndrome. J Cataract Refract Surg 2001;27(4):528-532.
[CrossRef]
2 Ritch
R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol 2001;45(4):265-315. [CrossRef]
3 Elgin
U, Şen E, Şimşek T, Tekin K, Yılmazbaş P. Early postoperative effects of
cataract surgery on anterior segment parameters in primary open-angle glaucoma
and pseudoexfoliation glaucoma. Turk J
Ophthalmol 2016;46(3):95-98. [CrossRef]
4
Hasegawa Y, Nejima R, Mori Y, Sakisaka T, Minami K, Miyata K, Oshika T. Risk
factors for corneal endothelial cell loss by cataract surgery in eyes with
pseudoexfoliation syndrome. Clin
Ophthalmol 2016;10: 1685-1689. [CrossRef] [PMC free article]
[PubMed]
5
Shingleton BJ, Neo YN, Cvintal V, Shaikh AM, Liberman P, O'Donoghue MW. Outcome
of phacoemulsification and intraocular lens implantion in eyes with
pseudoexfoliation and weak zonules. Acta
Ophthalmol 2017;95(2):182-187. [CrossRef]
[PubMed]
6
Tarkkanen AH, Kivelä TT. Comparison of primary open-angle glaucoma and
exfoliation glaucoma at diagnosis. Eur J
Ophthalmol 2015;25(2): 137-139. [CrossRef] [PubMed]
7
Hayashi K, Manabe S, Yoshimura K, Kondo H. Corneal endothelial damage after
cataract surgery in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg 2013;39(6):881-887. [CrossRef] [PubMed]
8 You
QS, Xu L, Wang YX, Yang H, Ma K, Li JJ, Zhang L, Jonas JB. Pseudoexfoliation:
normative data and associations: the Beijing eye study 2011. Ophthalmology 2013;120(8):1551-1558. [CrossRef] [PubMed]
9
Govetto A, Lorente R, Vázquez de Parga P, Rojas L, Moreno C, Lagoa F, Lorente
B. Frequency of pseudoexfoliation among patients scheduled for cataract
surgery. J Cataract Refract Surg 2015;41(6):1224-1231.
[CrossRef] [PubMed]
10
Shingleton BJ, Rosenberg RB, Teixeira R, O'Donoghue MW. Evaluation of
intraocular pressure in the immediate postoperative period after
phacoemulsification. J Cataract Refract
Surg 2007;33(11):1953-1957. [CrossRef] [PubMed]
11
Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation
syndrome. Am J Ophthalmol 2006;141(5):921-937.
[CrossRef] [PubMed]
12
Álvarez L, García M, González-Iglesias H, Escribano J, Rodríguez-Calvo PP,
Fernández-Vega L, Coca-Prados M. LOXL1 gene variants and their association with
pseudoexfoliation glaucoma (XFG) in Spanish patients. BMC Med Genet 2015;16:72. [CrossRef] [PMC free article]
[PubMed]
13
Drolsum L, Rongvold A, Nicolaissen B. Cataract and glaucoma surgery in
pseudoexfoliation syndrome: a review. Acta
Ophthalmol Scand 2007;85(8):810-821. [CrossRef] [PubMed]
14
Hayashi K, Hayashi H, Nakao F, Hayashi F. Changes in anterior chamber angle
width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology 2000;107(4):698-703. [CrossRef]
15 Lai
JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by
phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and
coexisting cataract; a prospective case series. J Glaucoma 2006;15(1):47-52. [CrossRef] [PubMed]
16
Mathalone N, Hyams M, Neiman S, Buckman G, Hod Y, Geyer O. Long-term
intraocular pressure control after clear corneal phacoemulsification in
glaucoma patients. J Cataract Refract
Surg 2005; 31(3):479-483. [CrossRef] [PubMed]
17 Chen
PP, Lin SC, Junk AK, Radhakrishnan S, Singh K, Chen TC. The effect of
phacoemulsification on intraocular pressure in glaucoma patients: a report by
the American Academy of Ophthalmology. Ophthalmology
2015;122(7):1294-1307. [CrossRef] [PubMed]
18 Guan
H, Mick A, Porco T, Dolan BJ. Preoperative factors associated with IOP
reduction after cataract surgery. Optom
Vis Sci 2013;90(2):179-184. [CrossRef] [PubMed]
19
Mierzejewski A, Eliks I, Kaluzny B, Zygulska M, Harasimowicz B, Kaluzny JJ.
Cataract phacoemulsification and intraocular pressure in glaucoma patients. Klin Oczna 2008;110(1-3):11-17. [PubMed]
20
Zetterström C, Behndig A, Kugelberg M, Montan P, Lundström M. Changes in
intraocular pressure after cataract surgery: analysis of the Swedish national
cataract register data. J Cataract
Refract Surg 2015;41(8):1725-1729. [CrossRef] [PubMed]
21
Shingleton BJ, Laul A, Nagao K, Wolff B, O’DonoghueM, Eagan E, Flattem N,
Desai-Bartoli S. Effect of phacoemulsification on intraocular pressure in eyes
with pseudoexfoliation: single-surgeon series. J Cataract Refract Surg 2008;34(11):1834-1841. [CrossRef] [PubMed]
22
Peräsalo R. Phaco-emulsification of cataract in eyes with glaucoma. Acta Ophthalmol Scand 1997;75(3):299-300. [CrossRef]
23
Moghimi S, Johari M, Mahmoudi A, Chen R, Mazloumi M, He M, Lin SC. Predictors
of intraocular pressure change after phacoemulsification in patients with
pseudoexfoliation syndrome. Br J
Ophthalmol 2017;101(3): 283-289.
24
Damji KF, Konstas AG, Liebmann JM, Hodge WG, Ziakas NG, Giannikakis S,
Mintsioulis G, Merkur A, Pan Y, Ritch R. Intraocular pressure following
phacoemulsification in patients with and without exfoliation syndrome: a 2 year
prospective study. Br J Ophthalmol 2006; 90(8):1014-1018. [CrossRef] [PMC free article]
[PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)