IF in JCR CiteScore
Rank About IJO Current
Issue Featured Articles Articles In Press Recent Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1396-1401
·Clinical Research·
··
Comparative analysis of cytomegalovirus retinitis
and microvascular retinopathy in patients with acquired immunodeficiency
syndrome
Chao Chen1,2, Chun-Gang Guo1,
Li Meng3, Jing Yu1, Lian-Yong Xie1, Hong-Wei
Dong1, Wen-Bin Wei2
1Beijing You
An Hospital, Capital Medical University, Beijing 100069, China
2Beijing
Tongren Eye Center, Beijing Key Laboratory of Intraocular Tumor Diagnosis and
Treatment, Beijing Ophthalmology and Visual Sciences Key Lab, Beijing Tongren
Hospital, Capital Medical University, Beijing 100730, China
3Xi'an Aier
Ancient City Eye Hospital, Xi'an 710021, Shaanxi Province, China
Correspondence
to: Wen-Bin Wei. Beijing Tongren Eye Center, Beijing Key Laboratory
of Intraocular Tumor Diagnosis and Treatment, Beijing Ophthalmology and Visual
Sciences Key Lab, Beijing Tongren Hospital, Capital Medical University, Beijing
100730, China. weiwenbintr@163.com
Received:
2017-04-05
Accepted: 2017-07-31
Abstract
AIM: To
compare the clinical manifestation of cytomegalovirus (CMV) retinitis and
microvascular retinopathy (MVR) in patients with acquired immunodeficiency
syndrome (AIDS) in China.
Methods: A total of 93
consecutive patients with AIDS, including 41 cases of CMV retinitis and 52
cases of MVR were retrospectively reviewed. Highly active antiretroviral
therapy (HAART) status was recorded. HIV and CMV immunoassay were also tested.
CD4+ T-lymphocyte count and blood CMV-DNA test were performed in all patients.
Aqueous humor CMV-DNA test was completed in 39 patients. Ophthalmological
examinations including best corrected visual acuity (BCVA, by International
Standard Vision Chart), intraocular pressure (IOP), slit-lamp biomicroscopy,
indirect ophthalmoscopy were performed.
Results: In
MVR group, the anterior segment examination was normal in all patients with a
mean BCVA of 0.93±0.13. Blood CMV-DNA was 0 (0, 269 000) and 42 patients
(80.77%) did not receive HAART. In CMV retinitis group, 13 patients (31.71%)
had anterior segment abnormality. The mean BCVA was 0.64±0.35 and blood CMV-DNA
was 3470 (0, 1 450 000). Nineteen patients (46.34%) had not received HAART. MVR
group and CMV retinitis group the positive rates of aqueous CMV-DNA were 0 and
50%, respectively. Two patients with MVR progressed to CMV retinitis during the
follow-up period.
Conclusion: In comparison of
CMV, patients with MVR have relatively mild visual function impairment. Careful
ophthalmological examination and close follow-up are mandatory, especially for patients
who have systemic complications, positive CMV-DNA test and without received
HAART.
KeyWords: acquired immunodeficiency syndrome;
cytomegalovirus retinitis; microvascular retinopathy; CD4+ T- lymphocyte;
CMV-DNA; highly active antiretroviral therapy
Citation: Chen C, Guo CG, Meng L, Yu J, Xie LY, Dong HW,
Wei WB. Comparative analysis of cytomegalovirus retinitis and microvascular
retinopathy in patients with acquired immunodeficiency syndrome. Int J
Ophthalmol 2017;10(9):1396-1401
Introduction
Acquired
immunodeficiency syndrome (AIDS) is caused by the human immunodeficiency virus
(HIV) which affects all body organs either directly or by opportunistic
infections, and the eye is not spared. AIDS is a multisystemic disease, but eye
diseases occur in up to 70% of the cases during the natural history of
infection. The spectrum of HIV-associated ophthalmic manifestations is very
broad and extends from a simple blepharitis to retinal abnormalities. Multiple
retinal abnormalities could be found in patients with AIDS. The most common
diseases include cytomegalovirus retinitis and microvascular retinopathy (MVR)[1]. Clinical manifestation of cytomegalovirus (CMV)
retinitis is a potentially blinding opportunistic infection that used to occur
in up to one-third of HIV-infected patients before the availability of Highly
active antiretroviral therapy (HAART). CMV retinitis is characterized by
typical white, crumbly areas of retinal necrosis and hemorrhage[2]. Cotton-wool spot is an early manifestation of CMV
retinitis. Previous studies have found that CMV-DNA is detected by polymerase
chain reaction (PCR) at the position of the cotton-wool spot[3].
CMV retinitis can cause irreversible vision loss and CMV retinitis is most
common reason for blindness in patients with AIDS[1].
HIV can also cause MVR assuming cotton-wool spots and small intraretinal
hemorrhage which leads to mild to moderate visual function impairment[4]. At some point the MVR may progress to CMV retinitis.
The fundamentals of CMV retinitis management are early diagnosis and specific
anti-CMV treatment. Up to now, there are rare studies reported about difference
and relationship between CMV retinitis and MVR in AIDS patients.
In this
study, we investigated 93 AIDS patients including 41 cases of CMV retinitis and
52 cases of MVR to analyze the clinical manifestation of CMV retinitis and MVR.
To the best of our knowledge, this is the comprehensive study to compare CMV
retinitis and MVR in AIDS patients in China.
sUBJECTS and Methods
Subjects This study
was approved by the Beijing You An Hospital, Capital Medical University Institutional
Review Board and adhered to the tenets of the Declaration of Helsinki. Records
of 93 consecutive patients with AIDS, including 86 males and 7 females, aging
from 21 to 65y were retrospectively reviewed. All patients had typical retinal
abnormality secondary to AIDS and visited the Ophthalmology Department of
Beijing You An Hospital, Capital Medical University between January 2013 and
April 2015. Among of them, 41 cases were diagnosed as CMV retinitis and 52
cases were diagnosed as MVR.
Examinations 1)
Ophthalmological examinations include best-corrected Snellen visual acuity
(BCVA) testing, intraocular pressure (IOP) measurement (Alcon®),
slit-lamp examination, indirect ophthalmoscopy, and Kowa VX-10 Fundus Camera
(Kowa Company, Ltd., Japan); 2) General examinations: HIV and blood CMV-DNA
diagnostic bioassay test were performed in all patients. CMV-DNA test of the
aqueous humor was recorded in 39 patients whose physical condition could
tolerate the examination. Among of them, the prevalence of MVR and CMV
retinitis was 48.7% (19 eyes) and 51.3% (20 eyes) respectively. HAART status of
all patients were recorded.
Diagnosis
Criteria 1) According
to the standard ACTGA criteria[5], diagnosis of
CMV retinitis was made by an experienced ophthalmologist based on the ocular
manifestation. The progress of CMV retinitis was recorded by fundus
photography. Exclusion criteria included other necrotizing retinitis induced by
varicella zoster virus, herpes simplex virus, syphilis, toxoplasmosis, or
lymphoma[6]. The uncertain cases should also be
ruled out; 2) MVR was diagnosed according to the typical retinal abnormality
such as cotton-wool spots, intraretinal hemorrhage, microaneurysms, and
ischemic maculopathy[6].
Data
Analysis Clinical
data of patients were analyzed using SPSS software for Windows (ver. 11.5,
SPSS, Inc., Chicago, IL, USA). Normal distributed quantitative data was
analyzed by t-test or ANOVA test. Qualitative data was analyzed by χ2
test or Fish’s exact probability method. For non-normal distributed data,
median of minimum and maximum value were recorded and analyzed by rank test.
Significance was defined as P<0.05.
Results
General
Information There were
41 patients including 37 males and 4 females in CMV retinitis group. The mean
patient age was 37.63±9.22y (range: 22 to 56y) . There were 52 patients
including 49 males and 3 females in MVR group. The mean subject age was
39.69±10.51y (range: 21 to 65y). There was no significant difference in the
mean age between CMV retinitis and MVR groups. Patients were followed range: 6
to 28mo. During the follow-up, two patients in MVR group developed status of
CMV retinitis.
Ocular and
Physical Examinations The
difference in BCVA, slit-lamp examination and blood CMV-DNA level between CMV
retinitis and MVR groups was statistically significant (Table 1). The mean BCVA
in MVR group was much better than that in CMV retinitis group (0.93±0.13 versus
0.64±0.35, t=-4.399, P=0.000). The slit-lamp examination was
normal anterior segment appearance in all patients in MVR group. In contrast,
13 patients (31.71%) in CMV retinitis group had abnormal anterior segment
appearance (t=19.167, P=0.000), including: ciliary hyperemia,
fine keratic precipitates, aqueous flare and cells. Blood CMV-DNA level was 0
(0, 269 000) in MVR group compared with 3470 (0, 1 450 000) in CMV retinitis
group (t=-3.377, P=0.001). The difference in IOP, CD4+
T-lymphocyte counts and blood HIV virus load was not statistically significant
between both groups (P>0.05).
Table 1 Ocular
and physical examinations

Aqueous
Humor CMV-DNA Analysis In this
study, 39 patients with systemic conditions could tolerate aqueous humor
CMV-DNA detection, including 19 people in MVR group, 20 people in CMV group.
CMV-DNA bioassay test was negative in all patients in MVR group, while 10
patients (50%) in CMV retinitis group had positive CMV-DNA test result (Table
2). The median value of CMV-DNA in the blood of MVR group was 0, the minimum
value was 0, the maximum detection value was 269 000, and the aqueous humor
CMV-DNA test was all 0. In the CMV group, the median CMV-DNA detection value
was 5215, the minimum value was 0, and the maximum detection value was 836 000.
The median of the measured values of CMV-DNA in the aqueous humor was 54.5, the
minimum was 0, and the maximum detected value was 1 650 000. The difference in
positive rate of blood and aqueous humor CMV-DNA test between the two groups
was statistically significant (t=-2.358, P=0.028; t=-3.478,
P=0.007). There were 3 patients in CMV retinitis group who had negative
blood CMV-DNA test results, while had positive aqueous humor CMV-DNA test
result.
Table 2
Blood and aqueous humor CMV-DNA analysis of 39 patients

HAART Status
and Analysis There were
10 patients (19.23%) in MVR group and 22 patients (53.66%) in CMV retinitis
group have received HAART in the first visit (Table 3). Patients without received
HAART were 42 (80.77%) in MVR group and 19 (46.34%) in CMV retinitis group. The
ratio of with HAART to without HAART in MVR and CMV retinitis group were 0.238
versus 1.158 respectively. The difference between both groups was statistically
significant (t=12.323, P=0.000).
Table 3
HAART treatment status in MVR and CMV groups
Two Patients
with MVR Developed CMV During the Follow-up Two patients
in MVR group were diagnosed as CMV retinitis during the follow-up (Figures
1-5). Their blood CD4+ T-lymphocytes were 20/µL and 1/µL respectively. The
common feature of these two patients was combining bacterial pneumonia and lung
tuberculosis. Both patients had positive blood CMV-DNA test that was 25 500 and
1 450 000 respectively. They had never received HAART before the first visit.
These two patients had poor compliance and did not follow-up closely.
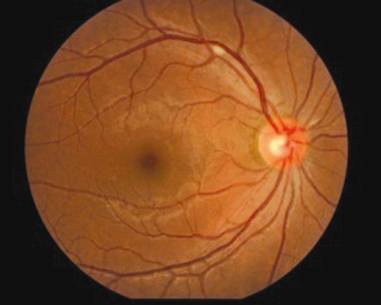
Figure 1
Initial fundus photograph from the patient who was diagnosed as MVR at the
first visit and developed CMV retinitis during the follow-up. Cotton-wool spot
was shown in the superotemporal retina of the right eye.
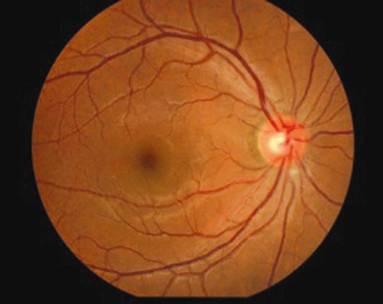
Figure 2
Fundus photograph of the same eye 3mo later demonstrated the cotton-wool spot
showing in Figure 1 disappeared with a new cotton-wool spot appearing under the
optic disc.

Figure 3
Fundus photograph of the same eye after 9mo displayed the typical CMV retinitis
changes with extensive retinal necrosis and hemorrhage.
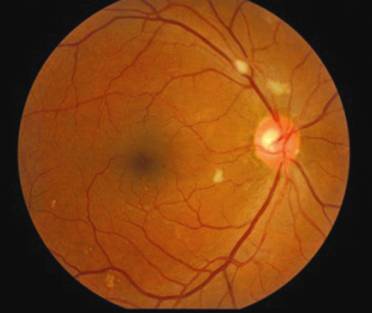
Figure 4
Fundus photograph at 3mo visit from the other patient who was diagnosed as MVR
initially and developed CMV retinitis during the follow-up. There were three
cotton-wool spots locating around the optic disc of the right eye.
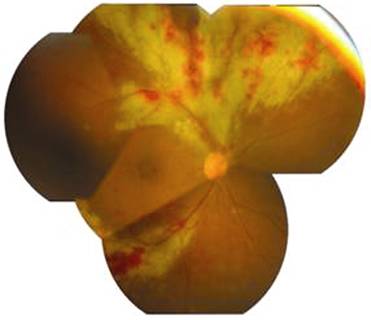
Figure 5
Fundus photograph of the same eye after 6mo follow-up visit showed superior and
inferotemporal retinal necrosis and hemorrhage indicating typical CMV retinitis
changes, whereas the initial cotton-wool spot disappeared.
Discussion
CMV
retinitis is the most common eye disease leading to blindness in AIDS
population. Early diagnosis for CMV retinitis could effectively control disease
progress, prevent other serious complications and retain the patient’s visual
function.
Previous
studies showed that low CD4+ T-lymphocyte level and HAART status was two major
risk factors for the onset of CMV retinitis and MVR in AIDS patients[6]. Another study indicated CD4+ T-lymphocyte count
<50/µL, without HAART, and less strict BCVA monitoring was associated with
CMV retinitis[2]. In our study, there was no
significant differences in CD4+ T-lymphocyte count between MVR and CMV
retinitis, while patients without HAART had a higher prevalence of MVR. In the
study of Abu et al[7], the same is no
significant association was found between ocular disorders and current CD4
counts. In accordance with previous studies, the mean BCVA of patients in CMV retinitis
group was lower than that of patients in MVR group[2].
Blood
CMV-DNA bioassay method was regarded to have lower sensitivity comparing with
other laboratory testing methods, therefore it was usually limited to CMV
retinitis diagnosis in clinical practice[8]. One
previous study reported blood CMV-DNA tests using PCR method could efficiently
support the diagnosis of mixed infection in HIV and CMV[4].
Our study revealed that CMV-DNA level in blood and aqueous humor samples in CMV
retinitis group was significantly higher than that in MVR group. This result
indicated that patients with high blood CMV-DNA virus load should be highly
suspect of CMV retinitis. It’s reported that CMV-DNA can also be found in
aqueous humor and vitreous samples[9]. And another
study found that CMV-DNA from both vitreous and aqueous specimens can provide
highly sensitive and specific markers to differentiate active and inactive CMV
retinitis[10].
In our
study, the positive rate of aqueous humor CMV-DNA between CMV group and MVR
group were 50% and 0 respectively. Aqueous humor CMV-DNA test could be helpful
to differentiate between CMV retinitis and MVR in AIDS patients. Totally
50%-70% of AIDS patients had MVR which manifested cotton-wool spots and retinal
bleeding[11]. Pathological changes of MVR are
pericyte loss and endothelial cell swelling[12].
Cotton-wool spots are the most common sign which can be found in 25%-50% AIDS
patients and in 75% of autopsy samples[13].
Active CMV retinitis is characterized by progressive white areas of retinal
necrosis and edema, with small white satellite lesions at the leading edge of
the active retinitis[14]. Lesions have often been
classified into an indolent/granular form or a fulminant/edematous form,
although the severity of opacity may be a more useful clinical description of
disease[15-16]. In practice,
it is usually difficult to identify the tiny retinopathy which is an early
manifestation of CMV retinitis or MVR in AIDS patients. In one of previous
study reported that CMV retinitis often happened in AIDS patients who had MVR
or already had MVR[17-18]. PCR
test of autopsy retina also showed CMV-DNA could be found in cotton-wool spot[17,19]. The above-mentioned proofs
supported the hypothesis of correlation between CMV infection and retinal blood
vessel damage[20-21]. In the
Johns Hopkins CMV Retinitis Cohort Study, the presence of HIV microangiopathy,
as evidenced by cotton-wool spots, increased the odds of CMV retinitis
1.46-fold (P<0.05)[22]. MVR usually
lasts for 6 to 12wk without impacting patient’s visual function and generally
do not need treatment, but should be regularly reviewed, and found that the
possible occurrence of CMV retinitis. Treatments for CMV retinitis include
intravenous ganciclovir, the oral pro-drug valganciclovir, intravenous
foscarnet, intravenous cidofovir, intravitreal injections of ganciclovir or
foscarnet, and the ganciclovir implant[23]. With
the effective anti-CMV treatment, CMV retinitis lesions began to dissipate,
clinically manifested as bleeding, exudation disappeared, retinal vein sheath
disappeared, and ultimately the formation of inactive scars. If retinal
detachment occurs, surgical treatment is usually required.
In our
study, there were two MVR patients progressed to CMV retinitis during the
follow-up. In the fundus lesion, four in five cotton spots have progressed into
CMV retinitis. The common features of these two patients were combining
systemic complications, poor compliance, positive blood CMV-DNA test and
HAART-naive status. Considering their poor physical condition, aqueous humor
CMV-DNA was not performed. In future study, blood and aqueous humor CMV-DNA
will be tested at different stage to further observe the relationship between
MVR and CMV retinitis.
The main
shortcoming of our study was limitation of retrospective research and that only
a few patients underwent aqueous humor CMV-DNA test. In addition, the mean
follow-up was only 13mo. Previous reports have indicated that retinal lesions
progressed over time and with longer AIDS history [24-25].
In
conclusion, the present study suggested the incidence of MVR was higher in AIDS
patients without HAART. CMV-DNA level of blood and aqueous humor in MVR group
were significantly lower than that in CMV retinitis group. Comparing with MVR,
CMV retinitis presented a significant vision-threatening problem. Aqueous humor
CMV-DNA test is helpful to differentiate between CMV retinitis and MVR. For MVR
patients with systemic complications, positive blood CMV-DNA test, and
HAART-naive status, careful initial ophthalmological examination and long-term
follow-up are mandatory.
ACKNOWLEDGEMENTS
Foundations: Supported
by National Natural Science Foundation of China (No.81570891;81272981); Beijing
Natural Science Foundation (No.7151003); Capital Medical University Fundamental
Clinical Research Cooperation Fund (No.16JL73); Beijing Municipal
Administration of Hospitals’ Ascent Plan (No.DFL20150201).
Conflicts of
Interest: Chen C, None; Guo CG, None; Meng L, None; Yu
J, None; Xie LY, None; Dong HW, None; Wei WB, None.
ReferenceS
1 Stewart MW. Optimal management of cytomegalovirus
retinitis in patients with AIDS. Clin
Ophthalmol 2010;4:285-299. [CrossRef]
[PubMed]
2
Sun HY, Peng XY, Li D, Mao FF, You QS, Jonas JB. Cytomegalovirus retinitis in
patients with AIDS before and after introduction of HAART in China. Eur J Ophthalmol 2014;24(2):209-215. [CrossRef] [PubMed]
3
González CR, Wiley CA, Arevalo JF, Garciá RF, Kuppermann BD, Berry C, Freeman
WR. Polymerase chain reaction detection of cytomegalovirus and human
immunodeficiency virus-1 in the retina of patients with acquired immune
deficiency syndrome with and without cotton-wool spots. Retina 1996;16(4):305-311. [CrossRef]
4
Tsen CL, Chen SC, Chen YS, Sheu SJ. Uveitis as an initial manifestation of
acquired immunodeficiency syndrome. Int J
STD AIDS 2017: 956462417694569. [CrossRef]
5
Mizushima D, Nishijima T, Yashiro S, Teruya K, Kikuchi Y, Katai N, Oka S,
Gatanaga H. Diagnostic utility of quantitative plasma cytomegalovirus DNA PCR
for cytomegalovirus end-organ diseases in patients with HIV-1 infection. J Acquir Immune Defic Syndr 2015;68(2):
140-146. [CrossRef]
[PubMed]
6
Becker KN, Becker NM. Ocular manifestations seen in HIV. Dis Mon 2014;60(6):268-275. [CrossRef] [PubMed]
7 Abu EK, Abokyi S, Obiri-Yeboah D, Ephraim RK, Afedo D,
Agyeman LD, Boadi-Kusi SB. Retinal microvasculopathy is common in HIV/AIDS
patients: a cross-sectional study at the Cape Coast Teaching Hospital, ghana. J Ophthalmol 2016;2016:8614095.
8
Jabs DA, Martin BK, Forman MS, Ricks MO; Cytomegalovirus Retinitis and Viral
Resistance Research Group. Cytomegalovirus (CMV) blood DNA load, CMV retinitis
progression, and occurrence of resistant CMV in patients with CMV retinitis. J Infect Dis 2005;192(4):640-649. [CrossRef] [PubMed]
9
Carmichael A. Cytomegalovirus and the eye. Eye
(Lond) 2012;26(2): 237-240. [CrossRef] [PMC free article]
[PubMed]
10
Sittivarakul W, Seepongphun U. Incidence rates and risk factors for vision loss
among AIDS-related cytomegalovirus retinitis patients in southern thailand. Ocul Immunol Inflamm 2017:1-8. [CrossRef] [PubMed]
11
Luo J, Jing D, Kozak I, Huiming Z, Siying C, Yezhen Y, Xin Q, Luosheng T,
Adelman RA, Forster SH. Prevalence of ocular manifestations of HIV/AIDS in the
highly active antiretroviral therapy (HAART) era: a different spectrum in
central south China. Ophthalmic Epidemiol
2013;20(3):170-175. [CrossRef]
[PubMed]
12
Glasgow BJ. Evidence for breaches of the retinal vasculature in acquired immune
deficiency syndrome angiopathy. A fluorescent microsphere study. Ophthalmology 1997;104(5):753-760. [CrossRef]
13
Mansour AM, Rodenko G, Dutt R. Half-life of cotton-wool spots in the acquired
immunodeficiency syndrome. Int J STD AIDS
1990;1(2): 132-133. [CrossRef]
[PubMed]
14
Heiden D, Ford N, Wilson D, Rodriguez WR, Margolis T, Janssens B, Bedelu M, Tun
N, Goemaere E, Saranchuk P, Sabapathy K, Smithuis F, Luyirika E, Drew WL.
Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med 2007;4(12):e334. [CrossRef] [PMC free article]
[PubMed]
15
Holland GN, Vaudaux JD, Jeng SM, Yu F, Goldenberg DT, Folz IC, Cumberland WG,
McCannel CA, Helm CJ, Hardy WD; UCLA CMV Retinitis Study Group. Characteristics
of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era
of highly active antiretroviral therapy (1988 to 1994). <ii>Am J
Ophthalmol</ii> 2008;145(1):5-11. [CrossRef] [PubMed]
16
Holland GN, Shuler JD. Progression rates of cytomegalovirus retinopathy in
ganciclovir-treated and untreated patients. <ii>Arch
Ophthalmol</ii> 1992;110(10):1435-1442. [CrossRef] [PubMed]
17
Mao F, Wu J, Sun H, You Q, Li D. Frosted branch angiitis in an AIDS patient
with cytomegalovirus retinitis. <ii>Int J Infect Dis</ii>
2016;52:9-11. [CrossRef]
[PubMed]
18
Freeman WR, Chen A, Henderly DE, Levine AM, Luttrull JK, Urrea PT, Arthur J,
Rasheed S, Cohen JL, Neuberg D. Prevalence and significance of acquired
immunodeficiency syndrome-related retinal microvasculopathy. <ii>Am J
Ophthalmol</ii> 1989;107(3):229-235. [CrossRef]
19
Yoganathan KT, Morgan AR, Yoganathan KG. Perforation of the bowel due to
cytomegalovirus infection in a man with AIDS: surgery is not always necessary!
<ii>BMJ Case Rep</ii> 2016;2016.pii:bcr2015214196.
20
Liu Y, Chen AS, Kamphaengkham S, Leenasirimakul P, Jirawison C, Ausayakhun S,
Margolis TP, Keenan JD. Diagnostic utility of ocular symptoms and vision for
cytomegalovirus retinitis. <ii>PLoS One</ii> 2016;11(10):e0165564.
[CrossRef] [PMC free article]
[PubMed]
21
Larochelle MB, Phan R, Craddock J, Abzug MJ, Curtis D, Robinson CC, Giller RH,
Cosgrove S, Siringo F, McCourt E, Palestine AG. Cytomegalovirus retinitis in
pediatric stem cell transplants: report of a recent cluster and the development
of a screening protocol. <ii>Am J Ophthalmol</ii> 2017;175:8-15. [CrossRef] [PubMed]
22
Jabs DA. Cytomegalovirus retinitis and the acquired immunodeficiency
syndrome--bench to bedside: LXVII Edward Jackson Memorial Lecture. <ii>Am
J Ophthalmol</ii> 2011;151(2):198-216.e1. [CrossRef] [PMC free article]
[PubMed]
23
Jabs DA, Ahuja A, Van Natta M, Dunn JP, Yeh S; Studies of the Ocular
Complications of AIDS Research Group. Comparison of treatment regimens for
cytomegalovirus retinitis in patients with AIDS in the era of highly active
antiretroviral therapy. <ii>Ophthalmology</ii>
2013;120(6):1262-1270. [CrossRef]
[PMC free
article] [PubMed]
24
Grønborg HL, Jespersen S, Hønge BL, Jensen-Fangel S, Wejse C. Review of
cytomegalovirus coinfection in HIV-infected individuals in Africa.
<ii>Rev Med Virol</ii> 2017;27(1):e1907. [CrossRef] [PubMed]
25
Iyer JV, Agrawal R, Yeo TK, Gunasekeran DV, Balne PK, Lee B, Au VB, Connolly J,
Teoh SC. Dataset of aqueous humor cytokine profile in HIV patients with
Cytomegalovirus (CMV) retinitis. <ii>Data Brief</ii> 2016;8:
1232-1242. [CrossRef] [PMC free article]
[PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)