IF in JCR CiteScore
Rank About IJO Current
Issue Featured Articles Articles In Press Recent Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1430-1435
·Meta-Analysis·
Diabetes and risk of glaucoma: systematic review and a Meta-analysis of
prospective cohort studies
Ying-Xi Zhao, Xiang-Wu Chen
Department
of Outpatient Service, the Eye Hospital of Wenzhou Medical University, Wenzhou
310020, Zhejiang Province, China
Correspondence
to: Xiang-Wu Chen. Department of Outpatient Service, the Eye
Hospital of Wenzhou Medical University, Wenzhou 310020, Zhejiang Province,
China. chenxiangwu_fd@sina.com
Received:
2016-12-19
Accepted: 2017-06-26
Abstract
AIM: To
quantify the association between diabetes and glaucoma using Meta-analysis.
METHODS: PubMed
and Embase were searched using medical subject headings and key words related
to diabetes and glaucoma. The inclusion criteria were: 1) the study design was
a prospective cohort study; 2) the exposure of interest was diabetes; 3) the
outcome of interest was primary open angle glaucoma (POAG); 4) risk ratios (RR)
and the corresponding 95% confidence interval (CI). Data were pooled using
fixed effects models to take into account heterogeneity between studies. Seven
prospective studies were selected. Diabetes increased the incidence of glaucoma
by 36% (OR=1.36, 95% CI=1.25-1.50). There was no evidence of statistical
heterogeneity (I2=0, P=0.53) or publication bias (the
funnel plot did not identify obvious asymmetry).
RESULTS:
Seven prospective cohort studies were incorporated in this Meta-analysis. The
pooled RR of the association between POAG and diabetes based on the risk
estimates of the seven cohort studies was 1.36 (95%CI=1.24-1.50), with no
significant heterogeneity across studies (I2=0; P=0.526).
The sensitivity analysis yielded a range of RRs from 1.34 (95%CI=1.22-1.48)
to1.40 (95%CI=1.18-1.67).
CONCLUSION: Diabetes
is associated with a significantly increased risk of glaucoma.
KEYWORDS: primary open angle glaucoma; diabetes; prospective studies
Citation: Zhao YX, Chen XW. Diabetes and risk of glaucoma: systematic review and a
Meta-analysis of prospective cohort studies. Int J Ophthalmol
2017;10(9):1430-1435
INTRODUCTION
Glaucoma is
a progressive optic disease that is mainly caused by high pressure in the eyes
and is characterized by gradual death of retinal ganglion cells (RGC)[1]. This eye disease, which is a leading cause of irreversible
blindness worldwide, has generated a major public health problem[2].Primary open angle glaucoma (POAG) is the most common
type of glaucoma in diabetic individuals, with nearly 70 million affected
worldwide[3]. Therefore, potential risk factors for
POAG need to be identified so that interventions to reduce its incidence can be
developed.
So far, the
pathogenesis of POAG is still not well understood. Some researches postulated
damage to the microvasculature network and/or reduced nutritional supply to the
RGC axons due to interference of blood regulation in the optic nerve head area[4-5]. This nutritional deficiency may
lead to degeneration of RGCs and initiate glaucomatous impairment. Therefore,
any vascular-related systemic disease, such as diabetes, which directly or
indirectly disrupts nutritional supply to RGCs, may result in development of
POAG.
Diabetes had
been deemed as a risk factor for POAG by some reports, however, epidemiologic
studies of the relationship between diabetes and POAG are still controversial.
Two previous Meta-analyzes found a statistically significant association
between diabetes and glaucoma[6-7].
However, most of the studies included in those Meta-analyzes were cross
sectional or case control, which were prone to more biases than prospective
studies. With recent accumulation of evidence, this review aimed to evaluate
the association of diabetes with POAG by performing a Meta-analysis of
prospective cohort studies.
MATERIALS
AND METHODS
Search
Strategy This
systematic review and Meta-analysis was reported following the guideline of
Meta-analysis of Observational Studies in Epidemiology. The protocol of this
systematic review was registered in the PROSPERO (No.CRD42016053714). PubMed
and Embase database were searched up to November 2016 for relevant studies that
tested the association between diabetes and glaucoma. The following search
terms were used: 1) diabetes mellitus, diabetes, glycuresis, risk factors; 2)
glaucoma, glaucomas; 3) cohort studies, prospective studies, cohort and
prospective. There will be no language restrictions. In addition, we manually
searched the reference lists from key articles and identified additional
relevant studies.
Study
Selection Our purpose
was to identify all studies reporting an association between diabetes and
glaucoma. We first conducted an initial screening of abstracts and titles. Then
a second screening based on full-text review was followed. Studies were
included in this Meta-analysis if they met the following criteria: 1) the study
design was a prospective cohort study; 2) the exposure of interest was
diabetes; 3) the outcome of interest was POAG; and 4) risk ratio (RR) or odds
ratio (OR) and the corresponding 95% confidence interval (CI) (or data to
calculate them) were reported. If more than one identified articles reported on
the same study population, we selected the study with the longest follow-up or
the most recent study.
Data
Extraction Two authors
(Chen XW and Zhao YX) separately reviewed all searched articles to determine
eligible studies and extracted data from selected results. Any disagreements
were resolved by discussion. Data extraction was performed by using a
standardized data-collection form. Information was extracted as follows: the
first author's last name; publication year; country location; characteristics
of study population (age, number of participants); number of POAG; methods for
identification of diabetes; RR (or OR) from the most fully adjusted models for
the diabetes compared with the non-diabetes and its corresponding 95% CI; and
statistical adjustments for confounding factors.
Quality
Assessment The study
quality was assessed by the Newcastle-Ottawa Scale, which is a star system that
comprised eight items to evaluate a study based on three broad perspectives,
including selection, comparability, and outcome categories. A study can be
awarded a maximum of one star for each numbered item within the selection and
outcome categories and a maximum of two stars for each item in the
comparability category. A score of five or higher indicated that the study had
high quality. A score of four or lower indicated that the study had low
quality. Two authors independently scored each included study and any
disagreement was resolved by discussion.
Statistical
Analyses The statistical
analyses were based on estimates extracted from prospective studies. Thus, RRs
and its 95% CI were used as the common measure of association across studies,
and were pooled within a Meta-analysis using the random effects model, which
were used for studies with considerable heterogeneity, or the fixed effects
model, which were used for studies with low heterogeneity. The hazard ratios
(HR) and OR were directly considered as RR for the incidence rate of glaucoma
was low.
The
homogeneity of RRs across studies was identified by using the Q
statistic (significance level at P<0.1). Furthermore, the value of I2
statistic, which was a quantitative measure of homogeneity across studies, was
calculated to provide a better interpretation of inconsistency across the
included studies. The values of I2>75%, <75%, <50%,
and <25% represent considerable, substantial, moderate, and low
heterogeneity, respectively.
A
sensitivity analysis was performed to investigate the influence of a single
study on the overall RR by omitting one study in each turn. Potential
publication bias was qualitatively assessed by funnel plots and quantitatively
assessed by Egger’s regression test, the latter would not be conducted if the
included studies <10 cases. The Meta-analysis was performed using the Stata
version 12.0 software statistical analysis.
RESULTS
Literature
Search We initially
obtained a total of 1921 citations (1167 from PubMed and 754 from Embase). Of
these, we excluded 354 publications because these were duplicate reports and
1550 publications because these were reviews, case reports, outcome or exposure
studies not relevant to our analysis, conducted in a population with specific
condition, or not prospective studies. After full text review of the remaining
17 papers, we selected 7 papers that were considered for analysis[8-14]. The main reasons for exclusion
were inappropriate data for pooled analysis or ineligible sample population.
The flowchart of study selection is shown in Figure 1.

Figure 1
Flow chart of study selection.
Study
Characteristics Table 1
provides an overview of key characteristic of the eligible studies. The
included studies were published between 2000 and 2014. The studies varied in
size between 3222 and 2 182 315 subjects, with an overall sample size across
the studies of 2 445 203. The mean length of follow-up in prospective studies
ranged from 2 to 20y. Two studies adjusted for age only and five studies
controlled a set of normal risk factors for glaucoma, such as gender, age,
diabetes, smoking, myopia and so on. Additionally, only one study adjusted for
intraocular pressure (IOP).
Table 1 key
characteristics of the eligible studies

Quality
Assessment The process
of quality assessment of the included studies is shown in Figure 2. For the
items “selection of the non-exposed cohort” and “adequacy of follow-up of
cohorts,” all included studies were awarded a maximum star. For the items
“ascertainment of exposure” and “assessment of outcome,” only four studies were
awarded one star. In general, one study was scored only 4 stars[14], whereas each of the other six studies was scored at
least 5 stars (5, 6, 7, 7, 8, and 9 stars).
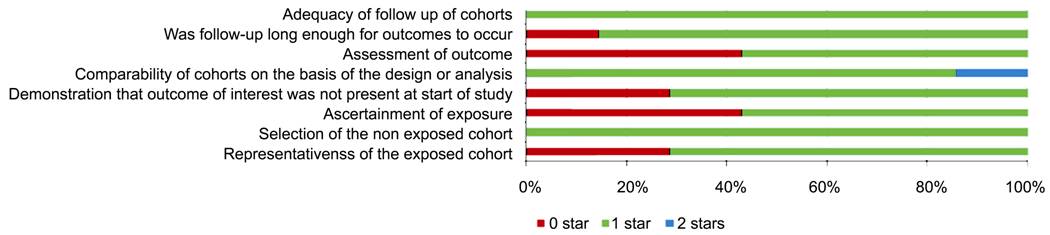
Figure 2
Methodological quality of included studies using the NOS tool.
Synthesis of
Results and Meta-analysis The pooled
RR of the association between POAG and diabetes based on the risk estimates of
the seven cohort studies was 1.36 (95% CI: 1.24-1.50), with no significant
heterogeneity across studies (I2 =0; P=0.526). These
results were showed in Figure 3. Of these 7 included studies, 3 studies found a
statistically significant association between diabetes and glaucoma and 4
studies found not.
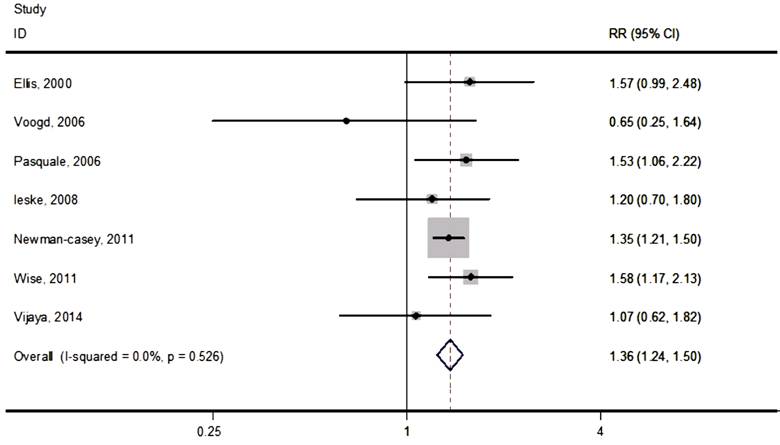
Figure 3
Forest plot of prospective cohort studies examining diabetes and risk of
glaucoma.
The
sensitivity analysis, which investigated the influence of a single study on the
overall risk estimate by omitting one study at each turn, yielded a range of
RRs from 1.34 (95%CI:1.22-1.48) to1.40 (95%CI:1.18-1.67). This suggested that
exclusion of any single study did not obviously alter the overall combined RR
(Figure 4).
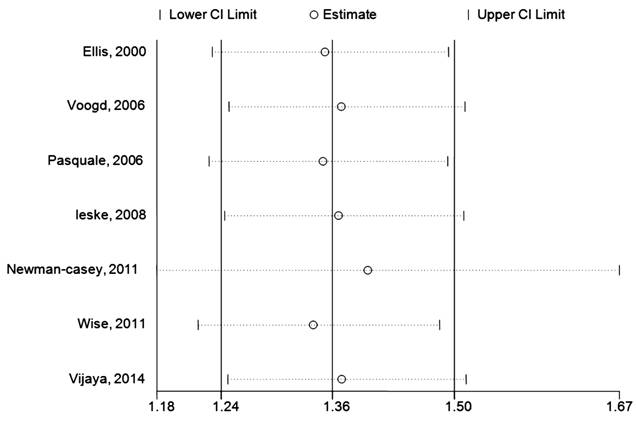
Figure 4
Sensitivity analysis of exclusion each single study.
Publication
Bias Visual
inspection of the funnel plot did not identify obvious asymmetry. The Egger
test for funnel plot asymmetry was not performed for that the power of
this test was too low to distinguish chance from real asymmetry when the
Meta-analysis included less than 10 studies (Figure 5).

Figure 5
Funnel plot of diabetes and risk of glaucoma incidence.
DISCUSSION
Diabetes
mellitus had been proposed as a risk factor for POAG, but epidemiologic studies
on the association between diabetes and glaucoma were still controversial.
Although some articles reported a positive association between diabetes and
glaucoma[11,15-18],
some others believed that the higher prevalence of glaucoma in individuals with
diabetes was caused by the more frequent ophthalmologic visits among diabetes
patients[13,19]. Two previous
Meta-analyses reported a positive association between POAG and diabetes[6-7]. However, the publication biases
reported in those systematic reviews were significant and a large number of nil
association studies were not incorporated in them[20-21]. Moreover, several cohort studies that had
accumulated in recent years were likewise not included[8-9]. Therefore, the direction and magnitude of pooled
estimates in these reviews should be interpreted with caution. To better
ascertain the association between diabetes and POAG, a more robust
Meta-analysis should be conducted.
In this
paper, we aimed to quantify the risk for development of glaucoma in individuals
with diabetes by performing a Meta-analysis of prospective cohort studies. The
results of this review revealed that the incidence of glaucoma markedly
increased by 36% (RR=1.36, 95%CI:1.25-1.50) in patients with diabetes compared with
individuals with no diabetes. In addition, the overall combined RRs were not
obviously altered by the exclusion of any single study in the sensitivity
analysis. Furthermore, we discovered that there was little heterogeneity in the
methods and quality of the original studies and the publication bias assessed
by the funnel plot in this review was not significant. To sum up, all these
findings provided strong evidence that there was a definitive association
between diabetes mellitus and POAG.
The
mechanisms relating diabetes to POAG were unclear. Several hypotheses on
biological links between diabetes mellitus and glaucoma had been proposed.
First, it was postulated that diabetes would lead to impairment of micrangium
and vascular autoregulation[22-24].
These vascular injuries would reduce blood flow to the retina and optic nerve[25-26], resulting in reduced nutrient
and oxygen supply to the RGC axons and increased expression of
hypoxia-inducible factor-1 in the retinal cells in response to elevated IOP.
Ultimately these was likely to induce the degeneration of the RGCs and
initiation of glaucomatous impairment. Second, there was a large amount of
evidence that the hyperglycemia and lipid anomalies induced by diabetes could
increase the risk of neuronal injury[4,27],
indicating that the RGCs were more likely to be killed in the patients with
diabetes. Third, the hyperglycemia of aqueous humor in the eyes of diabetes
patients would stimulate the synthesis and accumulation of fibronectin in the
trabecular meshwork to promote depletion of trabecular meshwork cells, which
could impair the outflow system of the aqueous humor and finally result in POAG[28-29].
A major
strength of this systematic review was that it was based on prospective cohort
studies, which minimized the possibility of selection and recall biases that
had always been the limitation of case control and cross sectional studies.
Another strength of this Meta-analysis was that all but one of the included
studies were scored as high quality, suggesting that there was little
methodological heterogeneity among the included studies. This point was
supported by the results of quantitative homogeneity assessment in this review
(I2=0, P>0.1). Finally, this Meta-analysis had a
larger sample size in the cohort studies as compared with two previous reviews,
revealing that the statistical power provided in this study was more precise
and reliable than the former Meta-analyses.
The
limitations of this Meta-analysis should be acknowledged when interpreting the
findings. First, the presence of residual confounders was always the concern of
prospective cohort studies. Although age, which was an important potential
confounding factor[30], was controlled in all
included studies, several other important potential confounding factors were
not sufficiently considered. For instance, IOP, which could affect the
relationship between diabetes and POAG, was not adjusted in all but one of the
selected studies. Therefore, the exclusion of likelihood factors that may be
responsible for the link between diabetes and glaucoma would weaken the
validity of this Meta-analysis. Second, there was considerable difference among
original studies with regard to population characteristics, follow-up years,
and diagnosis confirmations. These discrepancies would underestimate the
reliability of statistical results in the review. Third, the association
between glaucoma and type 1 diabetes may be different from that between
glaucoma and type 2 diabetes. Unfortunately, the type of diabetes in the included
studies was not detailedly described. This would limit the generalizability of
our findings. Finally, the quantitative assessment of publication bias was not
performed in this review for the inadequate included studies. Although visual
inspection of the funnel plot of the present Meta-analysis did not identify any
obvious asymmetry, indicating that there was no significant publication bias in
this review, the absence of the quantitative measurement of publication bias
would make the results in this review less convincible.
In
conclusion, the current Meta-analysis of prospective cohort studies provided
strong evidence in support of significant positive association between diabetes
and POAG. Yet the actual influence of important confounding factors, such as
IOP, central corneal thickness and so on, was not comprehensively investigated
in this review. Further prospective studies were warranted to clarify the role
of other important confounding factors in the diabetes and glaucoma
association.
ACKNOWLEDGEMENTS
Foundation: Supported by
the Plan of Wenzhou Science and Technology (No.Y20160439).
Conflicts of
Interest: Zhao YX, None; Chen XW, None.
REFERENCES
1 Quaranta
L, Riva I, Gerardi C, Oddone F, Floriano I, Konstas AG. Quality of life in
glaucoma: a review of the literature. Adv
Ther 2016; 33(6):959-981. [CrossRef] [PMC free article] [PubMed]
2 Actis AG,
Dall'Orto L, Penna R, Brogliatti B, Rolle T. An internal medicine perspective
review of risk factors for assessing and progression of primary open angle
glaucoma. Minerva Med
2013;104(4):471-485. [PubMed]
3 Resnikoff
S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti
SP. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82(11):844-851. [PMC free article] [PubMed]
4 Nakamura
M, Kanamori A, Negi A. Diabetes mellitus as a risk factor for glaucomatous
optic neuropathy. Ophthalmologica
2005;219(1):1-10. [CrossRef] [PubMed]
5 Kanamori
A, Nakamura M, Mukuno H, Maeda H, Negi A. Diabetes has an additive effect on
neural apoptosis in rat retina with chronically elevated intraocular pressure. Curr Eye Res 2004;28(1):47-54. [CrossRef] [PubMed]
6 Zhao D,
Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk
of glaucoma: a meta-analysis. Ophthalmology
2015;122(1):72-78. [CrossRef] [PubMed]
7 Zhou M,
Wang W, Huang W, Zhang X. Diabetes mellitus as a risk factor for open-angle glaucoma:
a systematic review and meta-analysis. PLoS
One 2014;9(8):e102972. [CrossRef] [PMC free article] [PubMed]
8 Leske MC,
Wu SY, Hennis A, Honkanen R, Nemesure B; BESs Study Group. Risk factors for
incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology 2008;115(1):85-93. [CrossRef] [PubMed]
9 Vijaya L,
Rashima A, Panday M, Choudhari NS, Ramesh SV, Lokapavani V, Boddupalli SD,
Sunil GT, George R. Predictors for incidence of primary open-angle glaucoma in
a South Indian population: the Chennai eye disease incidence study. Ophthalmology 2014;121(7): 1370-1376. [CrossRef] [PubMed]
10
Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between
components of metabolic syndrome and open-angle glaucoma. Ophthalmology 2011;118(7):1318-1326. [CrossRef]
11 Pasquale
LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE. Prospective study
of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology 2006;113(7): 1081-1086. [CrossRef] [PubMed]
12 de Voogd
S, Ikram MK, Wolfs RC, Jansonius NM, Witteman JC, Hofman A, de Jong PT. Is
diabetes mellitus a risk factor for open-angle glaucoma? The Rotterdam Study. Ophthalmology 2006;113(10):1827-1831. [CrossRef] [PubMed]
13 Ellis JD,
Evans JM, Ruta DA, Baines PS, Leese G, MacDonald TM, Morris AD. Glaucoma
incidence in an unselected cohort of diabetic patients: is diabetes mellitus a
risk factor for glaucoma? DARTS/MEMO collaboration. Diabetes Audit and Research
in Tayside Study. Medicines Monitoring Unit. Br J Ophthalmol 2000;84(11):1218-1224. [CrossRef] [PMC free article] [PubMed]
14 Wise LA,
Rosenberg L, Radin RG, Mattox C, Yang EB, Palmer JR, Seddon JM. A prospective study
of diabetes, lifestyle factors, and glaucoma among African-American women. Ann Epidemiol 2011;21(6): 430-439. [CrossRef] [PMC free article] [PubMed]
15 Mitchell
P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the Blue Mountains
eye study, Australia. Ophthalmology
1997;104(4):712-718. [CrossRef]
16 Chihara
E. Myopia and diabetes mellitus as modificatory factors of glaucomatous optic
neuropathy. Jpn J Ophthalmol
2014;58(1):16-25. [CrossRef] [PubMed]
17 Kawase K,
Tomidokoro A, Araie M, Iwase A, Yamamoto T; Tajimi Study Group; Japan Glaucoma
Society. Ocular and systemic factors related to intraocular pressure in
Japanese adults: the Tajimi study. Br J
Ophthalmol 2008;92(9):1175-1179. [CrossRef] [PubMed]
18 Hennis A,
Wu SY, Nemesure B, Leske MC; Barbados Eye Studies Group. Hypertension, diabetes,
and longitudinal changes in intraocular pressure. Ophthalmology 2003;110(5):908-914. [CrossRef]
19 Klein BE,
Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes. The Beaver
Dam Eye Study. Ophthalmology
1994;101(7): 1173-1177. [CrossRef]
20 Yamamoto
S, Sawaguchi S, Iwase A, Yamamoto T, Abe H, Tomita G, Tomidokoro A, Araie M.
Primary open-angle glaucoma in a population associated with high prevalence of
primary angle-closure glaucoma: the Kumejima Study. Ophthalmology 2014;121(8):1558-1565. [CrossRef] [PubMed]
21 Wang YX,
Xu L, Yang H, Jonas JB. Prevalence of glaucoma in North China: the Beijing Eye
Study. Am J Ophthalmol
2010;150(6):917-924. [CrossRef] [PubMed]
22 Quigley
HA. The pathogenesis of optic nerve damage in glaucoma. Trans New Orleans Acad Ophthalmol 1985;33:111-128. [PubMed]
23 Chopra V,
Varma R, Francis BA, Wu J, Torres M, Azen SP; Los Angeles Latino Eye Study Group.
Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles
Latino Eye Study. Ophthalmology
2008;115(2):227-232.e1. [CrossRef] [PMC free article] [PubMed]
24 Feke GT,
Bex PJ, Taylor CP, Rhee DJ, Turalba AV, Chen TC, Wand M, Pasquale LR. Effect of
brimonidine on retinal vascular autoregulation and short-term visual function
in normal tension glaucoma. Am J
Ophthalmol 2014;158(1):105-112.e1. [CrossRef] [PubMed]
25 Su DH,
Wong TY, Wong WL, Saw SM, Tan DT, Shen SY, Loon SC, Foster PJ, Aung T;
Singapore Malay Eye Study Group. Diabetes, hyperglycemia, and central corneal
thickness: the Singapore Malay Eye Study. Ophthalmology
2008;115(6):964-968.e1. [CrossRef] [PubMed]
26 Shami SK,
Chittenden SJ. Microangiopathy in diabetes mellitus: II. Features,
complications and investigation. Diabetes
Res 1991;17(4):157-168. [PubMed]
27
Bonaventure N, Roussel G, Wioland N. Stereospecificity of the action of
alpha-aminoadipic acid on the retina: a morphological and electrophysiological
study. C R Seances Acad Sci III
1983;296(9):457-462. [PubMed]
28 Yanagi M,
Kawasaki R, Wang JJ, Wong TY, Crowston J, Kiuchi Y. Vascular risk factors in
glaucoma: a review. Clin Exp Ophthalmol
2011; 39(3):252-258. [CrossRef] [PubMed]
29 Sato T,
Roy S. Effect of high glucose on fibronectin expression and cell proliferation
in trabecular meshwork cells. Invest
Ophthalmol Vis Sci 2002;43(1):170-175. [PubMed]
30 Reitmeir
P, Linkohr B, Heier M, Molnos S, Strobl R, Schulz H, Breier M, Faus T, Küster
DM, Wulff A, Grallert H, Grill E, Peters A, Graw J. Common eye diseases in
older adults of southern Germany: results from the KORA-Age study. Age Ageing 2016. [CrossRef] [PMC free article] [PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)