IF
in JCR CiteScore Rank About IJO Current Issue Featured
Articles Articles
In Press Recent
Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1446-1451
·Investigation·
Comparison of FDA safety and efficacy data for
KAMRA and Raindrop corneal inlays
Majid Moshirfar1,2, Jordan D Desautels1,3,
Ryan T Wallace4, Nicholas Koen5, Phillip C. Hoopes1
1HDR Research
Center, Hoopes Vision, Draper, Utah 84020, United States
2John A.
Moran Eye Center, Department of Ophthalmology and Visual Sciences, University
of Utah School of Medicine, Salt Lake City, Utah 84132, United States
3Tufts
University School of Medicine, Boston, Massachusetts 02111, United States
4Brigham
Young University, Provo, Utah 84602, United States
5Brown
University Alpert School of Medicine, Providence, Rhode Island 02906, United
States
Correspondence
to: Majid Moshirfar. HDR Research Center, Hoopes Vision, 11820 S.
State Street Suite #200, Draper, Utah 84020, United States. cornea2020@me.com
Received:
2017-02-09
Accepted: 2017-06-05
Abstract
Aim: To provide a
side-by-side analysis of the summary of safety and effectiveness data (SSED)
submitted to the FDA for the KAMRA and Raindrop corneal inlays for the
correction of presbyopia.
Methods: SSED reports
submitted to the FDA for KAMRA and Raindrop were compared with respect to loss
of corrected distance visual acuity (CDVA), adverse event rates, induction of
astigmatism, retention of contrast sensitivity, stability of manifest
refractive spherical equivalent (MRSE), and achieved monocular uncorrected near
visual acuity (UNVA) at 24mo.
Results: Totally 442/508
of KAMRA patients and 344/373 Raindrop patients remained enrolled in the
clinical trials at 24mo. The proportion of KAMRA and Raindrop patients who lost
≥2 lines of CDVA at 24mo was 3.4% and 1%, respectively. The adverse event rate
was comparable between the devices. No significant inductions of astigmatism
were noted. Both technologies induced a transient myopic shift in MRSE followed
by a hyperopic shift and subsequent stabilization. Totally 87% of KAMRA and 98%
of Raindrop patients attained a monocular UNVA of J5 (20/40) or better at 24mo,
28% of KAMRA and 67% of Raindrop patients attained a monocular UNVA of J1
(20/20) or better at 24mo.
Conclusion: Both devices
can be considered safe and effective, however, the results of corneal inlay
implantation are mixed, and long-term patient satisfaction will likely depend
on subjective expectations about the capabilities of the inlays. Variability in
surgical technique and postoperative care within and between the two clinical
trials diminishes the comparative power of this article.
Keywords: KAMRA; Raindrop; presbyopia; corneal inlay
Citation: Moshirfar M, Desautels JD, Wallace RT, Koen N, Hoopes PC. Comparison of
FDA safety and efficacy data for KAMRA and Raindrop corneal inlays. Int J
Ophthalmol 2017;10(9):1446-1451
Introduction
Presbyopia
describes an age related loss of near visual acuity with preserved distance
visual acuity. Although the exact mechanism of presbyopia has yet to be
elucidated, it is supposed that decreased compliance of the crystalline lens,
reduced function of extralenticular structures, and altered zonular tension due
to increases in lens diameter contribute to its occurrence[1-3]. It is estimated that by the year 2020, the worldwide
prevalence of presbyopia will rise to 1.4 billion[4]. Correspondingly, surgical
alternatives to spectacle correction, such as the implantation of Raindrop
(ReVision Optics, Lake Forest, CA, USA) and KAMRA (AcuFocus Inc., Irvine, CA,
USA) corneal inlays, are expected to attain a broader presence. Relevant
material properties, mechanisms of action, and surgical indications for both
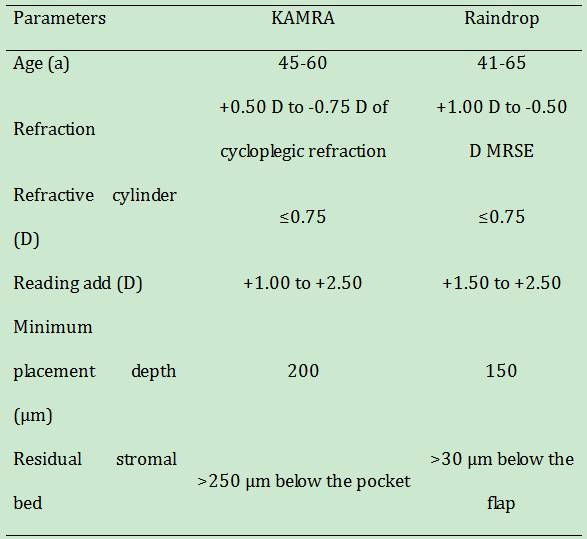
devices have been previously described, and are summarily presented in Tables 1
and 2[5].
In brief, the volume of the Raindrop inlay displaces anterior corneal
tissue into a steeper configuration that yields +1.5 to +2.5 diopters (D) of
near vision add. By contrast, the KAMRA inlay operates as a small aperture that
filters defocused peripheral rays to reduce the size of the retinal blur spot
in patients lacking sufficient accommodative amplitude.
Table 1
Device properties and mechanisms of action

Table 2
Surgical indications
Going
forward, it is important for refractive surgeons to have a comprehensive
understanding of the comparative safety and efficacy profiles of these devices
before offering them as treatment options. Although the summary of safety and
effectiveness data (SSED) reports from the pivotal FDA clinical trials are
available for both devices, these reports cannot be expediently compared due to
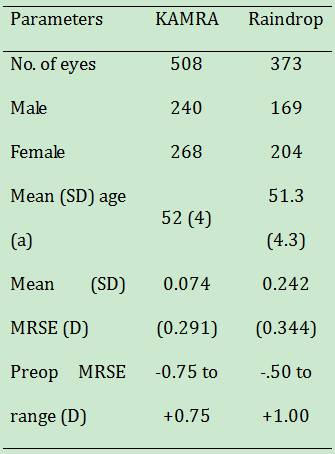
the use of differing outcome measures and data presentation[6-7]. A brief demographic comparison of the KAMRA and
Raindrop SSED reports is presented in Table 3. In this article, we seek to
compile the relevant safety and efficacy data for the KAMRA and Raindrop
corneal inlays in a standardized manner that eases comparison and facilitates
discussion with patients regarding surgical options for their presbyopia.
Table 3
Trial demographics
SUBJECTs AND Methods
This is a
comparative retrospective analysis of SSED reports pertaining to the pivotal
FDA clinical trials for the KAMRA and Raindrop corneal inlays. When possible,
safety and effectiveness outcomes were compared at 24mo postoperatively due to
sample size deterioration in both reports at 36mo and beyond. Cumulative data
pertaining to adverse event rates is presented through 36mo for both studies.
Comparative categories are as follows: obtainment of primary safety outcomes,
obtainment of secondary safety outcomes, stability, and efficacy.
We treat
having less than 5% of patients losing ≥2 lines of corrected distance visual
acuity (CDVA) 2y after inlay implantation, and at all subsequent visits, as the
primary safety criterion. Our secondary safety criteria include that less than
1% of eyes with a preoperative CDVA of 20/20 or better should have a CDVA worse
than 20/40 2y after surgery and beyond, less than 5% of eyes should have an
increase in manifest refractive astigmatism greater than 2.00 D from their
preoperative astigmatism at 2y and beyond, and the cumulative number of
surgically induced adverse events should be limited to 5% of eyes, with no more
than 1% of eyes experiencing any single surgically related adverse event over
the lifetime of the study. Adverse events with a clear causal link to inlay
implantation, such as significant loss of visual acuity, need for surgical
reintervention, and complications related to the implantation interface are
regarded as surgically induced adverse events. Although only the Raindrop
report treats refractive stability as a safety parameter, we include refractive
stability in our safety analysis due to its linkage to corneal changes. To be
considered stable, at least 95% of eyes should have ≤1.0 D of change in
manifest refractive spherical equivalent (MRSE) between any two refractions
performed at least 3mo apart. Moreover, the annualized mean rate of change in
MRSE should be ≤0.5 D (0.04 D/mo) between two refractions performed at least
3mo apart, and the mean rate of change in MRSE should level out to a rate that
is either explained by aging, or has a 95% confidence interval (CI) that
includes zero. Our standardized effectiveness criteria is related to the change
in monocular uncorrected near visual acuity (UNVA) in the implanted eye, and is
considered met when at least 75% of eyes achieve a CDVA of 20/40 (J5) or better
at 24mo and all subsequent visits.
It should be
noted that some data on MRSE change derived from the pivotal clinical trials
for each device was withheld from the SSED reports and was instead published in
the professional use information guides. Professional use data may not undergo
the same degree of vetting as FDA-reviewed SSED data.
Results
Both KAMRA
and Raindrop meet the targeted safety parameter of having less than 5% of
patients losing ≥2 lines of CDVA 2y after implantation and beyond (Figure 1).
Overall, the incidence of ≥2 lines of CDVA loss is lower for the Raindrop inlay
than for the KAMRA inlay. Although both devices meet the safety criteria for
loss of CDVA, it should be noted that at 36mo, 13% of Raindrop patients and 18%
of KAMRA patients still experienced a loss of >1 line of CDVA. Instances of
eyes that were 20/20 preoperatively becoming 20/40 or worse postoperatively
were well below the 5% occurrence threshold for the KAMRA cohort, and
nonexistent for the Raindrop patients (Figure 2).
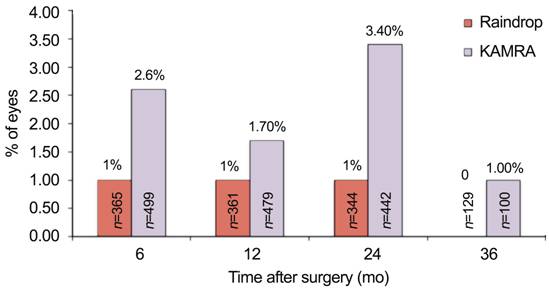
Figure 1
Percent of eyes showing a loss of two or more lines of CDVA at the given
postoperative time points
Of note, the sample size diminishes throughout the course of follow up
visits for both devices.
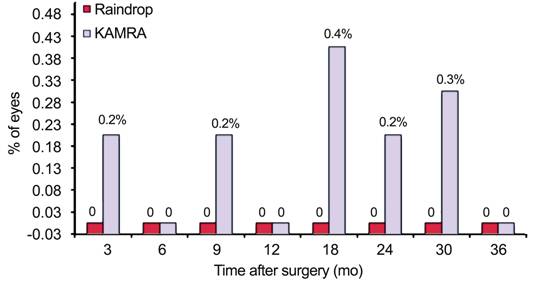
Figure 2
Percent of eyes with a preoperative CDVA of 20/40 or better that became worse
than 20/40 postoperatively.
No patients
in the Raindrop study experienced an induction of >2.00 D of manifest
refractive astigmatism at any time point. The percentage of KAMRA patients with
>2.00 D of induced manifest refractive astigmatism reached a maximum of 0.4%
at 9mo postoperatively before declining to 0.2% at 24mo, and 0.0% by 36mo.
The overall
adverse event rate is comparable for both devices (Table 4). Raindrop violated
the safety parameter that the cumulative surgically induced adverse event rate
should be less than 5% in that 44/373 (12%) eyes required secondary surgical
intervention (SSI) at some point at 3y following implantation. SSI’s include recentration,
explantation, additional refractive correction, epithelial ingrowth removal,
and lamellar interface rinse for diffuse lamellar keratitis (DLK). Totally
18/373 (5%) of Raindrop SSI’s were due to inlay exchange and 27/373 (7%) were
due to inlay explant. The predominant contributing factors to inlay explant
were corneal haze 10/27 (37%) and dissatisfaction with visual outcomes 10/27
(37%). The total incidence of corneal haze was 62/373 (17%) of eyes following
surgery. Therefore, approximately 16% of the experienced corneal haze was
severe enough to warrant explant. At the last available visit prior to explant,
the incidence of >1 line, ≥2 lines, and ≥3 lines of monocular UDVA loss
compared to baseline was 20/27 (74%), 16/27 (59%), and 11/27(41%) respectively.
Six months after explant, 5/18 (28%) eyes had persistent loss of >1 line,
3/18 (17%) eyes had a persistent loss of ≥2 lines, and no eyes had a persistent
loss of ≥3 lines of monocular UDVA. All patients had a CDVA of 20/20 or better
after explant. Decentration 2/27 (7%), epithelial ingrowth 2/27 (7%), and
patient request 3/27 (11%) were additional causes of inlay removal.
Furthermore, Raindrop exceeds the 1% occurrence threshold for singular adverse
events with regards to the rate of ocular infection 7/373 (2%), epithelial
ingrowth 10/373 (3%), cumulative loss of CDVA >2 lines at 3mo or later
11/373 (3%), increase in IOP >10 mm Hg above baseline 6/373 (2%), and DLK
6/373 (2%).
Table 4
Overall adverse event comparison between KAMRA and Raindrop n (%)

The KAMRA
inlay broke the 5% cumulative adverse event safety threshold in that 30/508
(6%) eyes experienced a loss of >2 lines of CDVA at 3mo or later and 55/508
(11%) eyes required SSI. SSI’s included epithelial ingrowth removal 3/508
(0.6%), lamellar interface rinse for DLK 1/508 (0.2%), explant 43/508 (8%),
recentration 6/508 (1%), and additional refractive correction 3/508 (0.6%). Of
the 43 inlays removed, 34/43 (79%) were prompted by visual complaints, with
hyperopic shift 24/34 (71%) being more common than myopic shift 2/34 (6%).
Totally 7/34 (20%) reported inadequate benefit, and 1/34 (3%) experienced
induced cylinder. An additional 2/43 (5%) of inlays were removed due to
cosmetic dissatisfaction. Totally 7/43 (16%) were removed secondary to medical
indications such as poor centration, persistant stromal opacity causing
sustained CDVA loss, inlay folding during implantation, stromal thinning due to
foreign body trauma, and posterior vitreous detachment and floaters in the
visual axis. KAMRA explantation did not shift CDVA by greater than one line
from baseline for any patients at the time of the last available follow up
visit. No data is available regarding monocular UDVA loss following explant.
KAMRA exceeds the 1% threshold for the occurrence of singular adverse events in
that 16/508 (3%) eyes experienced an increase in IOP >10 mm Hg from baseline
and 6/508 (1%) had postoperative DLK.
Monocular
contrast sensitivity with and without glare is mildly reduced after
implantation of both KAMRA and Raindrop, whereas binocular contrast sensitivity
is not appreciably affected. Although exact log contrast sensitivity values are
not supplied in either SSED report, the graphical data in each report supports
the notion that neither device provides a distinct advantage with respect to
the retention of contrast sensitivity.
Safety data
indicating the change in mean MRSE at the reported postoperative intervals is
shown in Figure 3. The Raindrop SSED report claims that >98% of eyes
experienced ≤1.00 D of MRSE change between all consecutive postoperative time
points. However, Raindrop MRSE change data is absent for the zero to 1mo
interval. KAMRA does not achieve a ≤1.00 D change in MRSE in ≥95% of patients
until the 9-12mo interval. This proportion then dips below the ≥95% threshold
until the 18-24mo interval.
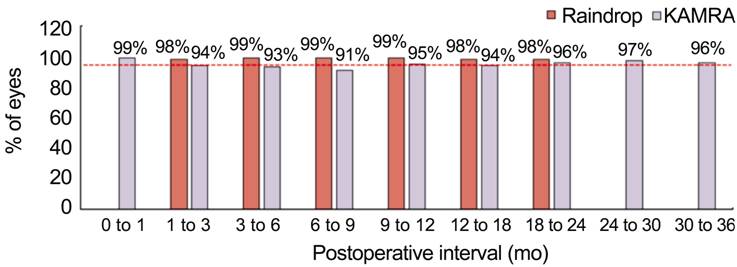
Figure 3
Percent of eyes experiencing a change in mean MRSE less than or equal to 1.0 D
between 3mo intervals up to 24mo Raindrop did not include data in
the ranges: 0-1mo and >24mo.
Approximate
changes in MRSE are shown in Figure 4 for qualitative purposes. The Raindrop
report presents a plot of mean MRSE between postoperative time points rather
than a list of the actual values, whereas the KAMRA report states all mean MRSE
values. As such, the mean change in MRSE for Raindrop can only be presented as
a near approximation. Overall, both devices experience a transient myopic shift
between zero and 3mo followed by a hyperopic shift and subsequent
stabilization. The magnitude of the zero to 1mo myopic shift is larger for
raindrop. Apparent stability is reached earlier for Raindrop (3 to 6mo) than
for KAMRA (6 to 9mo) as indicated by the fact that the 95% CI does not include
zero until the 9-12mo interval for KAMRA. The 95% CI includes zero at the
three-month time point and beyond for Raindrop. Raindrop data is not provided
beyond 24mo, however, KAMRA experiences a minor loss in MRSE in the 30-36mo
interval.
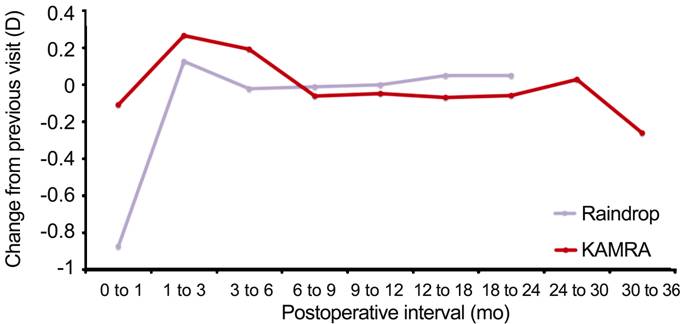
Figure 4
Change in MRSE between specified postoperative intervals.
Exact values
for the annualized mean rate of MRSE change between 3mo postoperative intervals
cannot be determined for Raindrop based on the provided study data. However,
the SSED data reports that the mean rate of MRSE change does not reach or
exceed 0.5 D/y between 3 and 24mo. The annualized mean rate of MRSE change for
KAMRA exceeds the ≤0.5 D/y threshold between the three and 6mo refractions, but
stabilizes below this threshold until the 30 to 36mo postoperative interval,
where the rate of MRSE change becomes -0.52 D/y (-0.043 D/mo).
Preoperatively,
0.3% (1/373) of Raindrop patients and 0 (0/508) of KAMRA patients had a UNVA of
J5 (20/40) or better. The results at 24mo for the KAMRA and Raindrop inlays are
shown in Figure 5. At 24mo, 87% (380/436) of KAMRA inlay patients and 92%
(336/364) of Raindrop patients achieved a UDVA of J5 (20/40) or better. Totally
120/432 (28%) of KAMRA and 230/344 (67%) of Raindrop patients had a UNVA of J1
(20/20) or better.
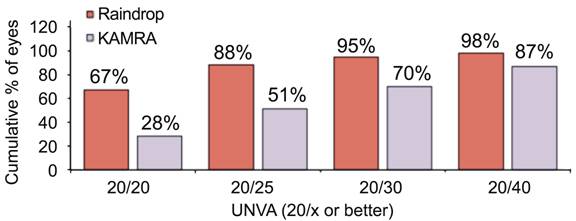
Figure 5
Efficacy results for monocular UNVA at 24mo.
Discussion
Both devices
met the safety criteria for postoperative loss of CDVA. Although the
comparative data cannot be statistically analyzed due to the inaccessibility of
the raw data, Raindrop does moderately outperform KAMRA in terms of the percentage
of patients who met the primary CDVA-loss safety parameter. It should be noted,
however, that CDVA loss after KAMRA is generally transient, and that only 5/442
(1%) of implanted eyes experienced a ≥2 lines CDVA loss that had persisted for
at least two consecutive visits by 24mo postoperatively. Additional studies
have corroborated high CDVA retention rates after KAMRA and Raindrop
implantation. In a prospective study of 57 KAMRA eyes, Moshirfar et al[8] report that no
eyes lost 2 or more lines of CDVA when implanting in an FS-laser pocket
generated with a 4×4 spot/line separation. Yilmaz et al[9] reported that
1/39 (3%) of eyes lost ≥2 lines of CDVA 6mo after KAMRA implantation under a
microkeratome flap. In a similar analysis of the raindrop inlay, Garza et al[10] showed that
0/20 Raindrop eyes experienced a loss of ≥2 lines of CDVA.
While tissue
healing responses and changes to the tear film are likely responsible for early
postoperative CDVA loss, the incidence peak at 24mo implicates other causes
related to mechanism of action, operative technique, and patient demographics.
One major mechanistic difference that likely yields better CDVA safety outcomes
for the Raindrop device is the multifocal effect imparted by the selective
steepening of the central cornea[10]. By contrast, defocused peripheral light rays are
either entirely excluded by the KAMRA inlay, or pass around the outer inlay
edge. A theoretical modeling study of KAMRA implanted eyes by Langenbucher et
al[11]
describes how peripheral rays that pass through and around the inlay may cast
retinal shadows that lead to reductions in contrast sensitivity and visual
acuity. However, neither the KAMRA nor Raindrop studies show significant
reductions in contrast sensitivity. Other authors have reported significant
improvements in binocular contrast sensitivity for near vision following KAMRA
implantation[12].
Baseline pupil diameter may also contribute to visual outcomes. Presumably, any
pupil with a diameter smaller than the outer KAMRA diameter (3.8 mm) will
result in passage of defocused peripheral light. A report by Tomita et al[13] regarding
visual acuity in 584 KAMRA implanted eyes showed that eyes with pupil diameters
>6.0 mm had significantly worse CDVA outcomes under mesopic conditions at
6mo postoperatively.
The higher
percentage of KAMRA inlay patients experiencing a loss of 2 or more lines of
CDVA could also stem from systematic and demographic differences within and
between the studies. MRSE, change in MRSE, and UNVA data from the KAMRA SSED
report that was stratified into 6×6 μm, 7×7 μm, 8×8 μm spot/line separtation,
or mechanical microkeratome lamellar resection methods revealed markedly better
outcomes in the 6×6 μm spot/line separation group. These results were further
confirmed by a follow up study that was appended to the SSED report. Although
subgroup specific data is not provided for CDVA, the correlation between
lamellar resection method and other visual outcome metrics implicates surgical
technique as a potential predictor of CDVA loss. Moreover, the Raindrop study
was performed on a group of eyes with a slightly hyperopic mean preoperative
MRSE (0.242±0.344). The steepening effect of the Raindrop may result in a
higher proportion of preoperative hyperopes becoming emmetropic postoperatively
than if the implant was placed in a group of eyes that was myopic on average.
The overall
adverse event rate is comparable for both technologies. Some effects, such as
elevated epithelial ingrowth following Raindrop compared to KAMRA,
postoperative intraocular pressure increases, and ocular infection are not
intrinsic to the devices, but rather to variability in implantation techniques
and postoperative management. Although we expect the creation of a flap for
Raindrop implantation to be associated with more corneal nerve damage and
subsequent dryness, KAMRA does not seem to produce any appreciable benefit in
terms of the occurrence of postoperative dry eye. Across both studies, only
1/373 (0.3%) Raindrop implanted eyes had severe persistent dry eye beyond 6mo
and 2/508 (0.4%) of KAMRA implanted eyes experienced diagnosable dry eye. Garza
et al[10]
present similarly minimal dry eye findings following Raindrop implantation, and
even showed improvement of dry eye in several eyes after Raindrop. The
relatively deep stromal placement depths for these devices may also limit
damage to the more anteriorly positioned sub-basal nerves.
SSI
resulting in inlay explantation is perhaps the most relevant adverse event from
a patient perspective. A crucial component of the decision making process for
inlay candidates should be an awareness that the overall SSI rate is near 10%
for both devices, and that approximately 8% (42/508) of KAMRA inlays and 7%
(27/373) of Raindrop inlays will require removal for various reasons. Explant
rates may have been particularly sensitive to individual trial sites that had
low thresholds for removal. This is particularly relevant for the KAMRA trials,
as they were performed across a larger variety of sites. It is further possible
that some inlay removals occurred outside of the study window. A large fraction
of removals will be prompted either by visual dissatisfaction resulting from an
induced loss of distance visual acuity or insignificant improvements in near
vision. Although the Raindrop report does not specify whether myopic or
hyperopic shift was a larger contributor to visual dissatisfaction, a large
majority of KAMRA patients had their inlays removed following a hyperopic
shift. Given that the KAMRA inlay has no refractive power, progressive
presbyopia will ultimately lead to hyperopic regression. By contrast, the
Raindrop inlay may provide a small buffer against presbyopic progression by
steepening the cornea to make patients slightly myopic. Prior data derived from
a limited subset of 10 KAMRA patients indicates that removal should occur
within the first 6mo to maximize visual and topographic outcomes[14].
Patients
should, however, expect fluctuations in visual acuity in the early
postoperative phase. Overall, the KAMRA data was more complete and transparent
in terms of providing the raw MRSE change data. As is expected based on the
corneal steepening effect, Raindrop patients are likely to experience a larger
myopic shift in the zero to one-month time frame, despite reaching refractive
stability earlier than KAMRA. Current literature regarding MRSE stability after
implantation is lacking for both inlays.
Both devices
met the efficacy benchmark of having at least 75% of eyes achieve a monocular
UNVA of 20/40 or better at 24mo. Although Raindrop appears to be more effective
in improving monocular UNVA, this metric may be more sensitive than any other
to surgical implantation technique and baseline patient characteristics.
Moshirfar et al[8]
reported that KAMRA inlays implanted at depths ≥250 µm resulted in 71% of
patients attaining a UNVA of 20/20 or better, whereas only 22% of patients with
inlays placed shallower than 250 µm experienced a UNVA of 20/20 or better.
Centration of the KAMRA inlay is also a subject of ongoing debate, and it is
not yet clear to what extent centering on the Purkinje reflex, pupil center, or
a point between affects visual outcomes. With regards to patient
characteristics, it has been shown that moderate baseline myopia is associated
with better visual outcomes after KAMRA implantation[15]. The KAMRA cohort involved in
the FDA trial had a mean refraction that was mildly hyperopic preoperatively
(0.074±0.291 D).
In summary,
although both inlays adequately met standardized measures of safety and
efficacy, patients should be presented with a realistic picture of the overall
rates of SSI, CDVA loss, and UDVA improvement. Differences in subject
demographics and surgical techniques diminish the comparative power of this article,
and emphasize the notion that results will likely be surgeon specific.
ACKNOWLEDGEMENTS
Conflicts of
Interest: Moshirfar M, None; Desautels JD, None; Wallace
RT, None; Koen N, None; Hoopes PC, holds shares in Acufocus
Inc.
References
1 Glasser A,
Campbell MC. Presbyopia and the optical changes in the human crystalline lens
with age. Vision Res
1998;38(2):209-229. [CrossRef]
2 Goldberg
DB. Computer animated model of accommodation and presbyopia. J Cataract Refract Surg 2016;41(2):437-445.
[CrossRef] [PubMed]
3 Schachar
RA. Cause and treatment of presbyopia with a method for increasing the
amplitude of accommodation. Ann
Ophthalmol 1992;24(12):445-447,452. [PubMed]
4 Holden BA,
Fricke TR, Ho SM, Wong R, Schlenther G, Cronjè S, Burnett A, Papas E, Naidoo
KS, Frick KD. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol 2008;126(12):1731-1739.
[CrossRef] [PubMed]
5 Arlt EM,
Krall EM, Moussa S, Grabner G, Dexl AK. Implantable inlay devices for
presbyopia: the evidence to date. Clin
Ophthalmol 2015;9:129-137. [PMC free
article] [PubMed]
9 Yilmaz OF,
Alagöz N, Pekel G, Azman E, Aksoy EF, Cakir H, Bozkurt E, Demirok A.
Intracorneal inlay to correct presbyopia: long-term results. J Cataract Refract Surg
2011;37(7):1275-1281. [CrossRef] [PubMed]
10 Garza EB,
Gomez S, Chayet A, Dishler J. One-year safety and efficacy results of a
hydrogel inlay to improve near vision in patients with emmetropic presbyopia. J Refract Surg 2013;29(3):166-172. [CrossRef] [PubMed]
11
Langenbucher A, Goebels S, Szentmáry N, Seitz B, Eppig T. Vignetting and field
of view with the KAMRA corneal inlay. Biomed
Res Int 2013;2013:154593. [CrossRef] [PMC free
article] [PubMed]
13 Tomita M,
Kanamori T, Waring GO 4th, Huseynova T. Retrospective evaluation of the
influence of pupil size on visual acuity after KAMRA inlay implantation. J Refract Surg 2014;30(7):448-453. [CrossRef] [PubMed]
14 Alio JL,
Abbouda A, Huseynli S, Knorz MC, Homs ME, Durrie DS. Removability of a small
aperture corneal inlay for presbyopia correction. J Refract Surg 2013;29(8):550-556. [CrossRef] [PubMed]
15 Tabernero
J, Artal P. Optical modeling of a corneal inlay in real eyes to increase depth
of focus: optimum centration and residual defocus. J Cataract Refract Surg 2012;38(2):270-277. [CrossRef] [PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)