IF in JCR CiteScore
Rank About IJO Current
Issue Featured Articles Articles In Press Recent Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1465-1473
·Review·
The role of elastic fibers in pathogenesis of
conjunctivochalasis
Jing-Yun Gan, Qing-Song Li, Zhen-Yong Zhang, Wei
Zhang, Xing-Ru Zhang
Department of Ophthalmology, Putuo Hospital, Shanghai University of
Traditional Chinese Medicine, Shanghai 200062, China
Correspondence to: Xing-Ru Zhang.
Department of Ophthalmology, Putuo Hospital, Shanghai University of Traditional
Chinese Medicine, Shanghai 200062, China. zhangxingru928@163.com
Received: 2016-11-22
Accepted: 2017-03-23
Abstract
The
PubMed, MEDLINE databases and China National Knowledge Infrastructure (CNKI)
were searched for information regarding the etiology and pathogenesis of
conjunctivochalasis (CCh) and the synthesis and degradation of elastic fibers.
After analysis of the literature, we found elastic fibers was a complex protein
molecule from the structure and composition; the degradation of elastic fibers
was one of the histopathological features of the disease; the vast majority of
the factors related to the pathogenesis of CCh ultimately pointed to abnormal
elastic fibers. By reasonably speculating, we considered that abnormal elastic
fibers cause the conjunctival relaxation. In conclusion, we hypothesize that
elastic fibers play an important role in the pathogenesis of CCh. Studies on
the mechanism of synthesis, degradation of elastic fibers are helpful to
clarify the pathogenesis of the disease and to find effective treatment
methods.
KEYWORDS: elastic fibers;
conjunctivochalasis; pathogenesis
Citation: Gan JY, Li QS, Zhang ZY, Zhang W, Zhang XR. The role of elastic fibers
in pathogenesis of conjunctivochalasis. Int J Ophthalmol 2017;10(9):1465-1473
INTRODUCTION
Cnjunctivochalasis (CCh), which was first proposed by Hughes[1] in 1942, defined as a redundant, loose, non-edematous
inferior bulbar conjunctiva interposed between the globe and the lower eyelid,
tends to be bilateral and is more prevalent in older populations. Extracellular
matrix which locates in substratum of epithelial or endothelial cells, around
connective tissue cells, provides mechanical support and physical strength to
the integrity of the organization, organ, and even the whole body. Under the
normal physiological conditions, elastic fibers are important components of
extracellular matrix, and influence the tissue flexibility and elasticity.
In 1998, Meller and Tseng[2] proposed the
hypothesis which states that mechanism of CCh is via accumulation of
degrading enzymes which resulted in elastotic degeneration and collagenolysis
of bulbar conjunctiva in the tears as a result of delayed tear clearance.
Although, a lot of research has been done on it, the precise etiology of
CCh remains obscure. So we summarize the previous literatures and analyze the
current situation on the hypothesis of elastic fibers.
MATERIALS AND METHODS
The following electronic databases were screened: PubMed, China National
Knowledge Infrastructure (CNKI). The following search equation was used: “CCh
(all fields)” OR “elastic tissue (MeSH terms)”, “elastic tissue (MeSH terms)”
AND “2011/1/1 (PDAT):2016/5/31 (PDAT)”. This equation was adapted to the
characteristics of each database. In addition we selected five newly published
papers, using their references to search the original literature. The last
search was on July 31, 2016.
SUMMARY OF ELASTIC FIBERS
Concept of Elastic Fibers Elastic fibers is a mainly connective tissue component of extracellular
matrix, produced by fibroblast, smooth muscle cells, some chondrocytes, giving
their organs, such as skin, lungs, arteries, ligamentum flavum, and auricle
cartilage with good flexibility.
Microscopic Behavior of Elastic Fibers Under the light microscope, the elastic fibers is presented as a fine
thread, and its branches are interwoven into a net, which is between 0.2 and 1 μm in diameter. Under electron microscope elastic fibers consist of an
amorphous elastin core surrounded by a mantle of longitudinally aligned
microfibrils in the mature state. Oxytalan is composed of 10-16 nm diameter
microtubules that are aligned along the fiber length. Elauninis formed from two
components, microtubules and amorphous material, which are considered to be an
immature state of elastic fibers[3].
Component of Elastic Fibers The major component of elastic fibers is elastin, which is formed following
the assembly and cross-linking of its soluble precursor, tropoelastin. Human
tropoelastin is encoded by a single gene that possesses 34 exons. The messenger
ribonucleic acid (mRNA) encodes a polypeptide depending on the splicing pattern
and removal of a signal peptide which leaves a mature protein (tropoelastin)[4]. Before it is released into the cell surface, elastin
binding protein (EBP) can be specifically combined with tropoelastin to prevent
it from polymerization and degradation of proteolytic enzymes in the cell[5].
The second component is visualized as small, 10-15 nm microfibrils that
localize to the periphery of the fiber in adult tissues and have a more complex
composition. The major structural element of microfibrils is contributed by the
fibrillins[6]. Numerous other proteins associate
with microfibrils or with elastin itself, including the microfibril associated
glycoproteins (MAGPs), fibulins and elastin microfibril interface located
protein-1 (EMILIN-1)[7]. Microfibrils is thought
to provide scaffold that facilitates elastin molecular alignment and subsequent
cross-linking, which is catalyzed by one or more members of the lysyl oxidase
(LOX) gene family[8].
Assembly and Degradation of Elastic Fibers The formation process of the elastic fibers is not completely clear.
Based on a large number of related studies, Wagenseil and Mechamt[9] proposed the model of elastic fibers assembly. First of
all, tropoelastin is transported to assembly sites on the plasma membrane where
it is organized into small aggregates that are cross-linked by a LOX. Secondly,
the aggregates remain on the cell surface while newly secreted elastin is added
to increase the size. The aggregates are then transferred to extracellular
microfibrils, which interact with the cell through integrins. Thirdly, elastin
aggregates on the microfibril coalesce into larger structures. At last, the
elastin aggregates are further cross-linked by LOX to form the complete elastic
fibers (Figure 1).
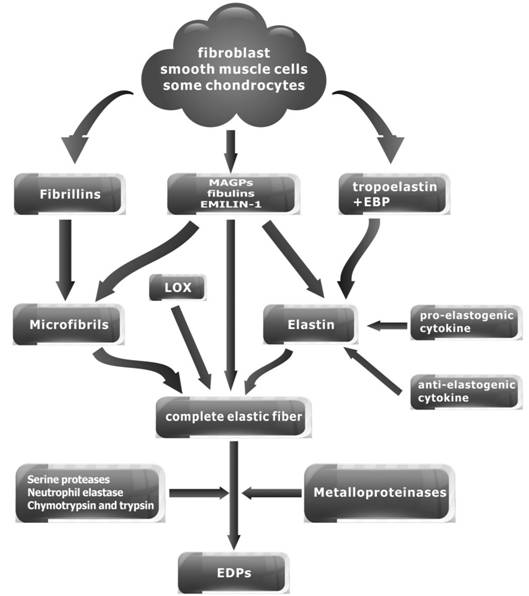
Figure 1 Summary of elastic fibers.
Research shows that intact microfibrils are effectively catabolised in
vitro by the serine proteases neutrophil elastase, chymotrypsin and trypsin[10]. In addition, fibrillin molecules and
fibrillin-rich microfibrils are degraded by matrix metalloproteinases (MMP-2,
MMP-3, MMP-9, MMP-12, MMP-13 and MMP-14) also[11].
Elastolysis by MMPs occur in development, wound healing, and major
inflammatory diseases. Elastin degradation by elastases generates
elastin-derived peptides (EDPs), which are highly chemotactic and stimulating
of inflammation, proliferation, and angiogenesis[12].
Cytokine Axis Regulates Elastin Formation and Degradation The formation and degradation of elastin was regulated by the cytokine
axis in which the pro-elastogenic activities of transforming growth factor β-1 (TGFβ1) and insulin-like growth factor-1 (IGF-1) are opposed by
anti-elastogenic activities of basic fibroblast growth factor (bFGF/FGF-2), heparin-binding
epidermal growth factor (HB-EGF)-like growth factor, epidermal growth factor (EGF)-like
growth factor, platelet derived growth factor-BB (PDGF-BB), transforming growth
factor-α (TGF-α), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and noncanonical TGFβ1 signaling[13]. It can be seen that the
elastic fibers is a complex protein molecules.
DEGRADATION OF ELASTIC FIBERS IS A HISTOPATHOLOGICAL FEATURES OF THE
CONJUNCTIVOCHALASIS
After reviewing the literature on the histopathology of the disease
associated with conjunctival relaxation (Table 1). From Table 1, we conclude
that the histopathological changes of the CCh are hyperplasia of squamous
epithelium with parakeratosis, infiltration of Inflammatory cells, decreased
collagen densities, degeneration of elastic fibers, dilated lymphatic vessels.
In fact, the histopathologic data on CCh are conflicting. For example, one
study showed that no significant difference in light microscopy findings
between eyes with CCh and those of age‑matched controls[14].
In spite of this, we can see that the degradation of elastic fibers is one of
the histopathological features of the disease.
Table 1 Histopathological changes of the CCh
|
Authors |
Time |
Country |
Results |
Staining |
Sample sizes |
|
Denti[15] |
1930 |
Italy |
Elastic tissue showed swelling, fragmentation, irregular course of the
fibers |
Weigert’s elastic-tissue stain |
Obscure |
|
Hughes[1] |
1942 |
USA |
No fragments and other abnormalities of elastic fibers were found |
Hematoxylin and eosin (H&E), Weigert’s elastic-tissue strain |
2, no control |
|
Watanabe et al[16] |
2004 |
Japan |
Negligible inflammation and lymphocyte infiltration, elastic fibers
fragmentation and sparsely assembled collagen fibers, 39/44 microscopic
lymphangiectasia |
Verhoeff-Van Gieson (VVG) staining |
44, no control |
|
Zhang et al[17] |
2004 |
China |
Hyperplasia of squamous epithelium with parakeratosis, pigmentation in
basal cell, hemorrhage and edema of stroma, infiltration of lymphocyte and
plasmocyte, decreased elastic fiber layer |
H&E, VVG staining, Masson trichrome staining, Mallory
phosphotungstic hematoxylin stain |
17 patients and 15
cataract |
|
Francis et al[18] |
2005 |
Australia |
Of 4 specimens (13.8%) had a chronic non-granulomatous inflammation
and 3 specimens (10.3%) demonstrated elastosis |
H&E staining, periodic acid Schiff, VVG staining |
29 patients and 24
cataract |
|
Ward et al[19] |
2010 |
Japan |
Decreased intercellular cohesiveness with an accumulation of elastic
fibers in conjunctival stroma |
VVG staining |
20 patients and 22
control |
|
Park et al[20] |
2011 |
Korea |
Decrease of collagen density, elastic degeneration, lymphangiectasia
in conjunctiva |
VVG staining, trichromestain, H&E staining |
27, no control |
|
Zhang et al[21] |
2013 |
China |
Elastic fibers decreased and melt of collagen fibers in lamina
propria, subconjunctival mild chronic inflammatory cell infiltration, visible
dilated lymphatics |
H&E staining, VVG staining, Masson trichromestaining, Mallory
phosphotungstic hematoxylin stain |
11, no control |
|
Bae and Park[22] |
2013 |
Korea |
Dilated lymphatic vessels, decreased goblet cell and collagen
densities, degeneration of elastic fibers |
H&E staining, VVG elastic staining |
14, no control |
|
Dong et al[23] |
2014 |
China |
Squamous epithelial hyperplasia, slight hyperkeratosis of some
superficial cells, pigment calm of basal cells, lamina propria vascular
congestion and expansion, interstitial infiltration of lymphoid and plasma
cells |
H&E staining |
20 patients and 22
control |
|
Kantaputra et al[24] |
2014 |
Thailand |
Hyperplasia of the conjunctival epithelium with subepithelial
conjunctival mononuclear inflammatory cell infiltration, very few blood
vessels with abnormal elastic fiber |
Silver staining |
1, no control |
|
Yu et al[25] |
2015 |
China |
Obvious squamous epithelial hyperplasia, parakeratosis, basal cell
pigmentation, lamina propria hemorrhage, infiltration of lymphocytes, and
reduction of elastic fibers and collagen fibers |
H&E staining, Masson’s
trichrome staining |
83, no control |
CAN THE DEGRADATION OF ELASTIN FIBERS CAUSE CONJUNCTIVOCHALASIS?
Analogical reasoning is a kind of common logic method. The logical form
of analogical reasoning is: A object and B object have attributes: a1,
a2, …, an; A object yet has attributes: an+1;
So, B object also has attributes: an+1.
Cutis laxa (CL) is characterized by a loose, redundant, hypoelastic
skin. Typically, the skin in CL can easily be pulled away from underlying
tissue and only slowly returns to its original position. Redundant skin is
often most noticeable on the neck, hands, and groin, but can also be seen on
the face, creating a premature aging appearance[26].
CCh is characterized by a redundant, loose, non-edematous inferior bulbar
conjunctiva interposed between the globe and the lower eyelid. It can occur in
the temporal, nasal, or middle of the conjunctiva.
The conjunctiva is a mucous membrane, which is composed of squamous
epithelium and goblet cells. It is divided into the epithelial layer and the lamina
propria, and the lamina propria is divided into the adenoid layer and the fiber
layer. The epithelium of the bulbar conjunctiva is a flat type, about 2-5, and
the fibrous layer is composed of collagen fibers and elastic fibers. On the
view of skin embryology, skin is composed of epidermis, dermis and subcutaneous
tissue and skin appendages. The dermis can be divided into papillary layer and
reticular layer, which mainly consists of collagen fiber, elastic fiber and
matrix. In terms of morphology, the fibrous layer of the bulbar conjunctiva is
equivalent to the reticular layer of the dermis.
As mentioned before, the degradation of elastic fibers is one of the
histopathological features of the CCh. In CL, microscopic findings include loss
of elaunin and sparse, fragmented elastic fibers in the reticular dermis. All
types of CL show some elastic abnormalities and no findings are specific for
individual types of CL[26].
Markedly increased MMPs (MMP-1, MMP-2, MMP-3, MMP-9, MMP-12), tissue
inhibitor of metalloproteinases-1 (TIMP-1) associated with the degradation of
elastic fibers and alteration of collagen fibers were found in CL by
immunohistochemistry[27]. Interestingly enough,
the expression levels of MMP-1, MMP-3, TIMP-1 were higher in CCh than those in
the control group, which were detected in the surgical specimen of the
conjunctiva by ELISA[28].
There is no effective drug treatment for CL, and surgical excision is
the main treatment method. Unlike persons with related connective tissue
disorders, patients with CL generally heal well after surgery. But surgical
treatment does not prevent the recurrence of skin relaxation, so patients often
require repeated surgery[29]. Tamura reported
botulinum toxin has been helpful in one case[30].
In the same way, surgical treatment is safe and effective for the treatment of
CCh, and it is necessary to select appropriate method according to the
condition of the patient and the classification of the disease. So far, the
medicine treatment can alleviate the symptom only. For example, regular use of
artificial tears can also improve the vision-related quality of life[31] and Pranoprofen Eye Drops can improve the patients
with grade II CCh with epiphora symptoms[32].
It is now clearly that inherited forms of CL involves genetic defects
which are elastin, fibulin (FBLN) 4, FBLN5, adenosine triphosphate (ATP) 6V0A2,
pyrroline-5-carboxylate reductase (PYCR) 1, ATP7A, solute carrier (SLC) 2A10,
latent TGF-binding protein (LTBP) 4, ras and rab interactor (RIN) 2. These
mutations eventually lead to elastic fiber synthesis disorders or functional
defects, causing CL. The pathogenesis of the CCh is also related to the genetic
defect, it is known that FBLN5 mutations lead to the disease. Kantaputra et
al[24] reported that a 4-year-old girl
suffering from autosomal recessive CL and CCh simultaneously.
The results showed that the histological structure of skin and
conjunctiva, and the genetic background, clinical manifestations, pathological
changes and treatment methods were very similar between CCh and CL. We know
that abnormal elastic fibers cause skin relaxation, so according to the
principle of analogical reasoning, to determine abnormal elastic fibers also
caused the conjunctival relaxation.
CAN FACTORS RELATED TO THE PATHOGENESIS OF CONJUNCTIVOCHALASIS LEAD TO
ABNORMAL ELASTIC FIBERS?
Genetic Factors Kantaputra et al[24] reported on a
4-year-old girl with autosomal recessive CL, type IA, or pulmonary emphysema
type, with loose and wrinkled skin, mitral and tricuspid valve prolapse, CCh,
obstructed nasolacrimal ducts, hypoplastic maxilla, and early childhood-onset
pulmonary emphysema. Histopathological study of the conjunctival biopsy showed
that most blood vessels had normal elastic fibers, hyperplasia of the
conjunctival epithelium with sub-epithelial conjunctival mononuclear
inflammatory cell infiltration. Mutation analysis of FBLN5 showed a homozygous
c.432C>G missense mutation, and heterozygosity in the parents. This is
predicted to cause amino acid substitution p.Cys144Trp[24].
This case report suggests that genetic factors are involved in the
pathogenesis of the CCh. FBLN5 is required to support LOX-mediated
cross-linking of elastin on the microfibrillar scaffold. FBLN5 mutations lead
to misfolding, decreased secretion, and a reduction of its interaction with
elastin and fibrillin-1 and eventually cause structure and functional
abnormalities of elastic fibers.
Mechanical Stress Clinical findings of CCh showed corneal margin type and introverted
lower eyelid type or corneal limbal type and introverted lower eyelid type, so
the author suggested high tension of lower eyelid is one factor of CCh
pathogenesis[33]. As mentioned above, dilated
lymphatic vessel is one of the histopathological features of the CCh. After the
clinical epidemiological survey, some scholars believe that bulbar conjunctival
lymphangiectasia may be one of the reasons for CCh[34].
Otaka and Kyu[35] hypothesize that the
decrease in connective tissue (elastotic degeneration and collagenolysis)
reduces the adhesion of bulbar conjunctiva to the eye, the conjunctiva is
“squeezed up” by the lower eyelid margin, and the conjunctival folds are formed
on the lower eyelid margin. Watanabe et al[16]
hypothesize that mechanical forces between the lower lid and conjunctiva gradually
interfered with lymphatic flow. Chronic, prolonged mechanical obstruction of
lymphatic flow may result in lymphatic dilation and eventually give rise to
clinical CCh[16]. High tension of lower eyelid
and lymphangiectasia constitute the CCh pathogenesis hypothesis of mechanical
stress.
Under the action of repeated mechanical forces, elastic fibers in the
aorta appeared fatigue fracture[36]. Previous
studies showed that the tensile stress can affect the extracellular matrix
metabolism of the organization, including MMPs and TIMP, gene expression and
protein synthesis of the matrix components. For example, under the action of
cyclic tensile, the expression of MMP-3, which was secreted in bovine synovial
cells seeded onto an artificial ligament scaffold, was up-regulated and the
enzyme activity was enhanced[37]. It can be seen
that mechanical stress lead to the destruction of elastic fibers through these
two pathways.
Apoptosis Factors Apoptosis regulatory protein, apoptosis related proteins and inflammatory
response associated protein was found in patients with CCh by tears for
proteomic analysis and was indicated that the incidence of CCh is related to
cell apoptosis and inflammation[38]. The collagen
fibril is decreased and fibroblast cells are degenerated in lamina and fascia
of CCh under the transmission electron microscope[39].
B cell lymphoma (Bcl)-2 and Bcl-2 associated X protein (Bax) are important gene
protein in regulating apoptosis and the ratio of them decided whether cells
survive after accepting the signal of apoptosis. The imbalance of expression of
the two in the conjunctival relaxation also confirmed the existence of
apoptosis[23].
Combined with the previous studies, we know that apoptosis occurs in
fibroblasts. The physiological function of fibroblasts is the synthesis of
extracellular matrix such as elastic fibers and collagen fibers. As a result
the conjunctival fibroblasts can not synthesize enough extracellular matrix to
compensate the degradation of its and leading to the formation of the disease.
Aging Factors Mimura et al[40] reported the
prevalence of CCh increased dramatically with age in a consecutive case study
including 1416 patients aged one to 94y. The severity of CCh affecting the
temporal and nasal bulbar conjunctiva was strongly correlated with age with
fourier-domain optical coherence tomography[41].
Among 2110 residents, 930 cases were confirmed as CCh, with a prevalence rate
of 44.08%. The prevalence rate increased with age[42].
Data show that CCh is a common age-related eye disease.
Tendons of old compared with young rats had decreased mRNA expression
levels of elastin[43]. Ageing has been shown to
enhance MMP-2, -7, -9, -14 activity in the aortic walls of rodents, non-human
primates, and humans[44]. The effect of aging on
the human body is reflected in the increased destruction and synthesis
deficiency of elastin.
Inflammatory Factors Wear contact lenses and autoimmune thyroid disease are important risk
factor for CCh. Contact lenses-induced CCh is probably attributable to
mechanically induced inflammation that is related to dryness and friction
between the lens and conjunctiva[45]. When suffer
from autoimmune thyroid disease, the body will be over express of inflammatory
factors[46].
In fact, excessive inflammatory factors were detected in tears from
patient. Wang et al[47] found that the
levels of inflammatory cytokines in tear of CCh are higher than in the normal
population, especially loose conjunctiva in nasal side. Erdogan-Poyraz et al[48] found that tear IL-6 and IL-8 levels are elevated in
patients with CCh yet. Higher IL levels are observed in advanced stages of the
disease, especially when punctal occlusion. What is more interesting is that
tear IL levels tend to parallel the clinical severity as evaluated with the
Ocular Surface Disease Index (OSDI). Inflammatory cell infiltration in tissue
samples of patients also supports the pathogenesis of inflammatory factors.
Meller et al[49] found IL-1β and TNF-α can up-regulate mRNA and protein
over-expression of MMP-1 and MMP-3 in cultured CCh fibroblasts in vitro.
Up-regulation and activation of MMPs by inflammatory factors lead to the
degradation of the elastic fibers of the conjunctiva.
Ultraviolet Radiation Pinguecula was independently associated with CCh after adjustment for
age. It has been clear that the pinguecula and the ultraviolet (UV) radiation
are related, so it is speculated that there is a relationship between the
conjunctival relaxation and the UV radiation[50].
The destruction of elastic fibers and UV radiation is linked. Photoaging
is the process by which natural sunlight and/or artificial sources of UV
radiation damage the skin. Histologically, changes can be observed in the
epidermis and dermis. Dermal changes include the hallmark of photoaged skin,
the so-called solar elastosis: this accumulated elastotic material in the mid-
and upper-dermis is most likely a breakdown product of elastic fibers[51]. It is known that UV radiation induces damage to skin
mainly by superfluous reactive oxygen species and chronic low-grade
inflammation, which eventually up-regulate the expression of MMPs[52].
Oxidative Stress Specimens from patients with CCh revealed a significantly higher number
of cells positively stained for hexanoyl-lysine (HEL),
8-hydroxy-2-deoxyguanosine (8-OHdG), MMP-3, and MMP-9 than the control
subjects. These findings revealed lipid and DNA oxidative stress were present
in the conjunctiva in patients with CCh[19].
Acera et al[53] have identified a group of
proteins, which is up-regulated in CCH tears. Some of them, such as calgranulin
(S100) A4, S100A8, and peroxiredoxin-5, are markers of inflammation and
oxidative processes. These studies suggest that the onset of the disease
involves oxidative stress.
Takayasu's arteritis (TA) is an inflammatory disorder characterized by
destruction of elastic fibers. Increased oxidative stress and MMPs activity
were considered to play an active role in the progression of TA disease[54]. Neutrophil elastases are thought to be central
players to the process of intrinsic skin aging and photoaging. Indeed, not only
they directly contribute to the direct degradation of elastic fibers under
oxidative stress but also, through a complex network of biochemical reactions,
their interferences with collagen homeostasis in skin and contribute, to some
extent, to exacerbate oxidative stress in skin[55].
Oxidative stress leads to degradation of elastic fibers through matrix
metalloproteinase and neutrophil elastase.
Other Factors
Refractive error and axial length
Mimura et al[56]
reported that the prevalence and grade of CCh are dependent on refractive error
and hyperopia being an important risk factor for the diseases. After two years
they suggest that the severity of CCh is dependent on the axial length (AL) and
a short AL contributing to the pathogenesis of CCh in another article[57].
Stress response Heat shock proteins (HSPs) are several families of proteins which are
synthesized by organisms inducing of stressors. HSPs are highly conserved, and
play an important role in the survival of stressed cells and stabilization of
internal environment. The research showed that the expression levels of HSP27
were higher in CCh than those in the control group[58].
The relationship between refractive error and AL, stress response and
elastic fibers were obscure. The vast majority of the factors related to the
pathogenesis of CCh ultimately point to abnormal elastic fibers, as the saying
goes: all roads lead to Rome (Figure 2).

Figure 2 Elastic fibers and the pathogenesis of CCh.
RESEARCH STATUS OF ELASTIC FIBERS IN NORMAL CONJUNCTIVA AND LOOSE
CONJUNCTIVA
Elastic Fibers in Normal Conjunctiva Scattered immature and very occasional mature elastic fibers were
observed in the stroma of the bulbar conjunctiva in young subjects (1-15y).
Oxytalan and lesser numbers of elaunin and mature elastic fibers intermingled
with the loose collagen bundles in both structures in older subjects (over
15y). The more elderly subjects had the most mature elastic tissue[59].
Elastic Fibers in Conjunctivochalasis Elastogenesis is restricted to foetal and infancy, and mature elastin
fibers remain for lifespan. Indeed, its strong reticulation makes elastin a
highly stable molecule with longevity comparable with human lifespan and any
proteolytic damage that does occur with age and disease is essentially
irreparable[60]. Under pathological conditions,
vascular and inflammatory cells can, however, produce tropoelastin, but these
tropoelastin molecules fail to cross-link into mature elastic fibers[61].
Based on the above understanding, the researchers focus on the
expression of MMPs in the loose conjunctiva. Li et al[62]
reported that overexpression of MMP-1 and MMP-3 mRNA by CCh cultured
fibroblasts is correlated with their increased protein levels and proteolytic
activities. All conjunctival resection specimens from the patients with CCh
revealed marked staining for MMP-3 and MMP-9, both in the epithelium and
conjunctival stroma compared with that in specimens obtained during cataract
surgery from the age- and sex-matched control subjects in the study of ward[19]. At the same time, the expression of MMPs in patients
with tear also enhanced. The concentration of pro-MMP-9 was significantly
higher in the CCh eyes than in the healthy controls[63].
Related studies also revealed that the activity of MMPs is regulated by the
inflammatory factors. Guo et al[64]
reported that act MMP-1 was uniquely found in cell lysates and culture media of
resting CCh fibroblasts, and such expression was further augmented by IL-1β in CCh fibroblasts. It is well known that MMPs can degrade collagen and
elastic fibers, so these data indirectly verified the hypothesis of the
previous[2].
The description of the elastic fibers appeared only in the results of
histological examination of the CCh. In addition, we have not seen the
literature on the elastic fibers of the CCh. So the research on elastic fibers
itself is ignored.
CONCLUSION
In conclusion, elastic fibers play an important role in the pathogenesis
of CCh. In the future, the research direction of the pathogenesis and
prevention and treatment of the disease should be put on the elastic fibers
that have been neglected. When the body is damaged, the compensatory mechanism
will play a role to repair the damage. For example, in the anemia of the body,
the bone marrow can be compensated to synthesize more red blood cells to
correct the anemia. It is worth to study whether there is compensatory
mechanism in elastic fibers damage.
As mentioned above, the elastogenesis is restricted to foetal and
infancy and mature elastic fibers remain for lifespan. When the elastic fibers
is damaged by age and disease, it can’t be repaired. Under pathological
conditions, vascular and inflammatory cells can produce tropoelastin, but these
tropoelastin molecules fail to cross-link into mature elastic fibers. The
mechanism of the above phenomenon has never been clarified.
In short, there are too many problems for elastic fibers. The answers to
the above questions can help us to clarify the pathogenesis of CCh and to find
an effective treatment for the disease.
ACKNOWLEDGEMENTS
Foundations: Supported by the Key
Medical Discipline Project of Shanghai Municipal Health Bureau- Ophthalmology
(No.ZK2015A20); the Health System Independent Innovation Science Foundation of
Shanghai Putuo District; Plateau Science,Integrated Traditional Chinese and Western
Medicine, Shanghai University of Traditional Chinese Medicine.
Conflicts of Interest: Gan JY, None; Li QS, None; Zhang ZY, None; Zhang W, None; Zhang
XR, None.
REFERENCES
1 Hughes WL. Conjunctivochalasis.
Am J Ophthalmol 1942;25:48-51. [CrossRef]
2 Meller D, Tseng SC. Conjunctivochalasis: literature review and
possible pathophysiology. Surv Ophthalmol
1998;43(3):225-232. [CrossRef]
3 Garner A, Alexander RA. Histochemistry of elastic and related fibres
in the human eye in health and disease. Histochem
J 1986;18(8):405-412. [CrossRef] [PubMed]
4 Wise SG, Weiss AS. Tropoelastin. Int
J Biochem Cell B 2009;41(3): 494-497. [CrossRef] [PubMed]
5 Hinek A, Braun KR, Liu K, Wang Y, Wight TN. Retrovirally mediated
overexpression of versican v3 reverses impaired elastogenesis and heightened
proliferation exhibited by fibroblasts from Costello syndrome and Hurler
disease patients. Am J Pathol 2004;164(1):119-131.
[CrossRef]
6 Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein,
is a component of extracellular microfibrils. J Cell Biol 1986;103(6 Pt 1): 2499-2509. [CrossRef] [PubMed]
7 Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci 2002;115(Pt 14):2817-2828. [PubMed]
8 Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector
of cell function. Cell Mol Life Sci 2006;63(19-20):2304-2316.
[CrossRef] [PubMed]
9 Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 2007;81(4):229-240.
[CrossRef] [PubMed]
10 Kielty CM, Woolley DE, Whittaker SP, Shuttleworth CA. Catabolism of
intact fibrillin microfibrils by neutrophil elastase, chymotrypsin and trypsin.
FEBS Lett 1994;351(1):85-89. [CrossRef]
11 Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth
CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: implications
for connective tissue remodelling. Biochem
J 1999;340(Pt 1): 171-181. [CrossRef] [PMC free article] [PubMed]
12 Antonicelli F, Bellon G, Debelle L, Hornebeck W. Elastin-elastases
and inflamm-aging. Curr Top Dev Biol 2007;79:99-155.
[CrossRef]
13 Sproul EP, Arqraves WS. A cytokine axis regulates elastin formation
and degradation. Matrix Biology 2013;32(2):86-94.
[CrossRef] [PMC free article] [PubMed]
14 Hashemian H, Mahbod M, Amoli FA, Kiarudi MY, Jabbarvand M, Kheirkhah
A. Histopathology of conjunctivochalasis compared to normal conjunctiva. J Ophthalmic Vis Res 2016;11(4):345-349.
[CrossRef] [PMC free article] [PubMed]
16 Watanabe A, Yokoi N, Kinoshita S, Hino Y, Tsuchhashi Y.
Clinicopathologic study of conjunctivochalasis. Cornea 2004;23(3): 294-298. [CrossRef]
17 Zhang XR, Cai RX, Wang BH, Li QS, Liu YX, Xu Y. The analysis of
histopathology of conjunctivochalasis. Zhonghua
Yan Ke Za Zhi 2004;
40(1):37-39. [PubMed]
18 Francis IC, Chan DG, Kim P, Wilcsek G, Filipic M, Yong J, Coroneo MT.
Case-controlled clinical and histopathological study of conjunctivochalasis. Br J Ophthalmol 2005;89(3):302-305. [CrossRef] [PMC free article] [PubMed]
19 Ward SK, Wakamatsu TH, Dogru M, Ibrahim OM, Kaido M, Ogawa Y,
Matsumoto Y, Igarashi A, Ishida R, Shimazaki J, Schnider C, Negishi K, Katakami
C, Tsubota K. The role of oxidative stress and inflammation in
conjunctivochalasis. Invest Ophthalmol
Vis Sci 2010;51(4):1994-2002. [CrossRef] [PubMed]
20 Park JS, Ha SW, Lew H. Histopathologic properties of eyelid skin and
conjunctiva in patients with dermatochalasis. J Korean Ophthalmol Soc 2011;52(5):582. [CrossRef]
21 Zhang XR, Zhang ZY, Hoffman MR, Li QS, Liu B, Zhou HM. The effect of
age and conjunctivochalasis on conjunctival thickness. Curr Eye Res 2013;38(3):331-334. [CrossRef] [PubMed]
22 Bae JB, Park WC. Histopathologic characteristics of
conjunctivochalasis. J Korean Ophthalmol
Soc 2013;54(8):1165. [CrossRef]
24 Kantaputra PN, Kaewgahya M, Wiwatwongwana A, Wiwatwongwana D,
Sittiwangkul R, Iamaroon A, Dejkhamron P. Cutis laxa with pulmonary emphysema,
conjunctivochalasis, nasolacrimal duct obstruction, abnormal hair, and a novel
FBLN5 mutation. Am J Med Genet A 2014;164A(9):
2370-2377. [CrossRef] [PubMed]
25 Yu XY, Jian ZY, Wu W, Lu XH. Simultaneous treatment of pterygium
complicated with conjunctivochalasis: analysis of pterygium excision and
conjunctival autotransplantation combined with sclera fixation. BMC Ophthalmol 2015;15:100. [CrossRef] [PMC free article] [PubMed]
26 Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutislaxa: a
review. J Am Acad Dermatol
2012;66(5):e1-17. [CrossRef] [PubMed]
27 Gu W, Liu W, Yang X, Tian Y, Meng R, Zhao Q. Cutis laxa: analysis of
metalloproteinases and extracellular matrix expression by immunohistochemistry
and histochemistry. Eur J Dermatol
2011;21(5):717-721. [PubMed]
29 Banks ND, Redett RJ, Mofid MZ, Manson PN. Cutis laxa: clinical
experience and outcomes. Plast Reconstr
Surg 2003;111(7):2434-2442. [CrossRef] [PubMed]
30 Tamura BM, Lourenco LM, Platt A, Pertel P, Santos LF, LevitesJ. Cutis
laxa: improvement of facial aesthetics by using botulinum toxin. Dermatol Surg 2004;30(12 Pt
2):1518-1520. [CrossRef]
31 Kiss HJ, Nemeth J. Isotonic glycerol and sodium hyaluronate
containing artificial tear decreases conjunctivochalasis after one and three
months: a self-controlled, unmasked study. PLoS
One 2015;10(7): e0132656. [CrossRef] [PMC free article] [PubMed]
34 Zhang XR, Liu YX, Sheng X, Zhou HM, Han ZM, Fu ZX, Li QS, Xiang MH.
Clinical observation of lymphangiectasis in conjunctivochalasis cases. Zhonghua Yan Ke Za Zhi 2013;49:(6):547-550.
[PubMed]
35 Otaka I, Kyu N. A new surgical technique for management of
conjunctivochalasis. Am J Ophthalmol 2000;129(3):385-387.
[CrossRef]
36 Duca L, Blaise S, Romier B, Laffargue M, Gavral S, El Btaouri H,
Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P. Matrix ageing and
vascular impacts: focus on elastin fragmentation. Cardiovasc Res 2016;110(3):298-308. [CrossRef] [PubMed]
37 Raif el M. Effect of cyclic tensile load on the regulation of the
expression of matrix metalloproteases (MMPs -1, -3) and structural components
in synovial cells. J Cell Mol Med 2008;12(6A):2439-2448. [CrossRef] [PMC free article] [PubMed]
38 Zhang XR, Xiang MH, Wu QQ, Li QS, Xu Y, Sun AG. The tear proteomics
analysis of conjunctivochalasis. Zhonghua
Yan Ke Za Zhi 2009;45(2):135-140. [PubMed]
40 Mimura T, Yamagami S, Usui T, Funatsu H, Mimura Y, Noma H, Honda N,
Amano S. Changes of conjunctivochalasis with age in a hospital-based study. Am J Ophthalmol 2009;147(1):171-177.e1.
[CrossRef] [PubMed]
41 Gumus K, Pflugfelder SC. Increasing prevalence and severity of
conjunctivochalasis with aging detected by anterior segment optical coherence
tomography. Am J Ophthalmol 2013;155(2):238-242.e2.
[CrossRef] [PubMed]
42 Zhang X, Li Q, Zou H, Peng J, Shi C, Zhou H, Zhang G, Xiang M, Li Y.
Assessing the severity of conjunctivochalasis in a senile population: a
community-based epidemiology study in Shanghai, China. BMC Public Health 2011;11:198. [CrossRef] [PMC free article] [PubMed]
43 Kostrominova TY, Brooks SV. Age-related changes in structure and
extracellular matrixprotein expression levels in rat tendons. Age (Dordr) 2013;35(6):2203-2214. [CrossRef] [PMC free article] [PubMed]
44 Wang M, Kim SH, Monticone RE, Lakatta EG. Matrix metalloproteinases
promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015;65(4):698-703. [CrossRef] [PMC free article] [PubMed]
45 Mimura T, Usui T, Yamamoto H, Yamagami S, Funatsu H, Noma H, Honda N,
Fukuoka S, Amano S. Conjunctivochalasis and contact lenses. Am J Ophthalmol 2009;148(1):20-25.e1. [CrossRef] [PubMed]
46 de Almeida SF, de Sousa LB, Vieira LA, Chiamollera MI, Barros Jde N.
Clinic-Cytologic Study of conjunctivochalasis and its relation to thyroid
autoimmune diseases. Cornea 2006;25(7):789-793.
[CrossRef] [PubMed]
47 Wang Y, Dogru M, Matsumoto Y, Ward SK, Ayako I, Hu Y, Okada N, Ogawa
Y, Shimazaki J, Tsubota K. The impact of nasal conjunctivochalasis on tear
functions and ocular surface findings. Am
J Ophthalmol 2007;144(6):930-937. [CrossRef] [PubMed]
48 Erdogan-Poyraz C, Mocan MC, Bozkurt B, Gariboglu S, Irkec M, Orhan M.
Elevated tear interleukin-6 and interleukin-8 levels in patients with
conjunctivochalasis. Cornea 2007;28(2):189-193.
[CrossRef] [PubMed]
49 Meller D, Li DQ, Tseng SC. Regulation of collagenase, stromelysin,
and gelatinase b in human conjunctival and conjunctivochalasis fibroblasts by
interleukin-1 and tumor necrosis factor-alpha. Invest Ophthalmol Vis Sci 2000;41(10):2922-2929. [PubMed]
50 Mimura T, Mori M, Obata H, Usui T, Yamagami S, Funatsu H, Noma H,
Amano S. Conjunctivochalasis: associations with pinguecula in a hospital-based
study. Acta Ophthalmol 2012;90(8):773-782.
[CrossRef] [PubMed]
51 Chen VL, Fleischmajer R, Schwartz E, Palaia M, Timpl R.
Immunochemistry of elastotic material in sun-damaged skin. J Invest Dermatol 1986;87(3):334-337. [CrossRef]
52 Zhang X, Xie YL, Yu XT, Su ZQ, Yuan J, Li YC, Su ZR, Zhan JY, Lai XP.
Protective effect of super-critical carbon dioxide fluid extract from flowers
and buds of chrysanthemum indicum Linnén against ultraviolet-induced
photo-aging in mice. Rejuvenation Res 2015;18(5):437-448. [CrossRef] [PMC free article] [PubMed]
53 Acera A, Suarez T, Rodriguez-Agirretxe I, Vecino E, Duran JA. Changes
in tear protein profile in patients with conjunctivochalasis. Cornea 2011;30(1):42-49. [CrossRef] [PubMed]
54 Mahajan N, Dhawan V, Malik S, Jain S. Implication of oxidative stress
and its correlation with activity of matrix metalloproteinases in patients with
Takayasu's arteritis disease. Int J
Cardiol 2010;145(2): 286-288. [CrossRef] [PubMed]
56 Mimura T, Usui T, Yamagami S, Funatsu H, Noma H, Toyono T, Mori M,
Amano S. Relationship between conjunctivochalasis and refractive error. Eye Contact Lens 2011;37(2):71-78. [CrossRef] [PubMed]
57 Mimura T, Yamagami S, Kamei Y, Goto M, Matsubara M. Influence of
axial length on conjunctivochalasis. Cornea
2013;32(8):1126-1130. [CrossRef] [PubMed]
59 Alexander RA, Garner A. Elastic and precursor fibres in the normal
human eye. Exp Eye Res 1983;36(2):305-315.
[CrossRef]
60 Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked
longevity of human lung parenchymal elastic fibers deduced from prevalence of
D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 1991;87(5):1828-1834. [CrossRef] [PMC free article] [PubMed]
61 Todorovich-Hunter L, Johnson DJ, Ranger P, Keeley FW, Rabinovitch M.
Altered elastin and collagen synthesis associated with progressive pulmonary
hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest 1988;58(2):184-195. [PubMed]
62 Li DQ, Meller D, Liu Y, Tseng SC. Overexpression of MMP-1 and MMP-3
by cultured conjunctivochalasis fibroblasts. Invest Ophthalmol Vis Sci 2000;41(2):404-410. [PubMed]
63 Acera A, Vecino E, Duran JA. Tear MMP-9 levels as a marker of ocular
surface inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci 2013;54(13):8285-8291. [CrossRef] [PubMed]
64 Guo P, Zhang SZ, He H, Zhu YT, Tseng SC. PTX3 controls activation of
matrix metalloproteinase 1 and apoptosis in conjunctivochalasis fibroblasts. Invest Ophthalmol Vis Sci 2012;53(7):3414-3423.
[CrossRef] [PMC free article] [PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)